Your shopping cart is currently empty
Your shopping cart is currently empty
Article | 09 Feb 2024
WIKIMOLE of the week—Bl-2493 & Bl-2865
By TargetMol
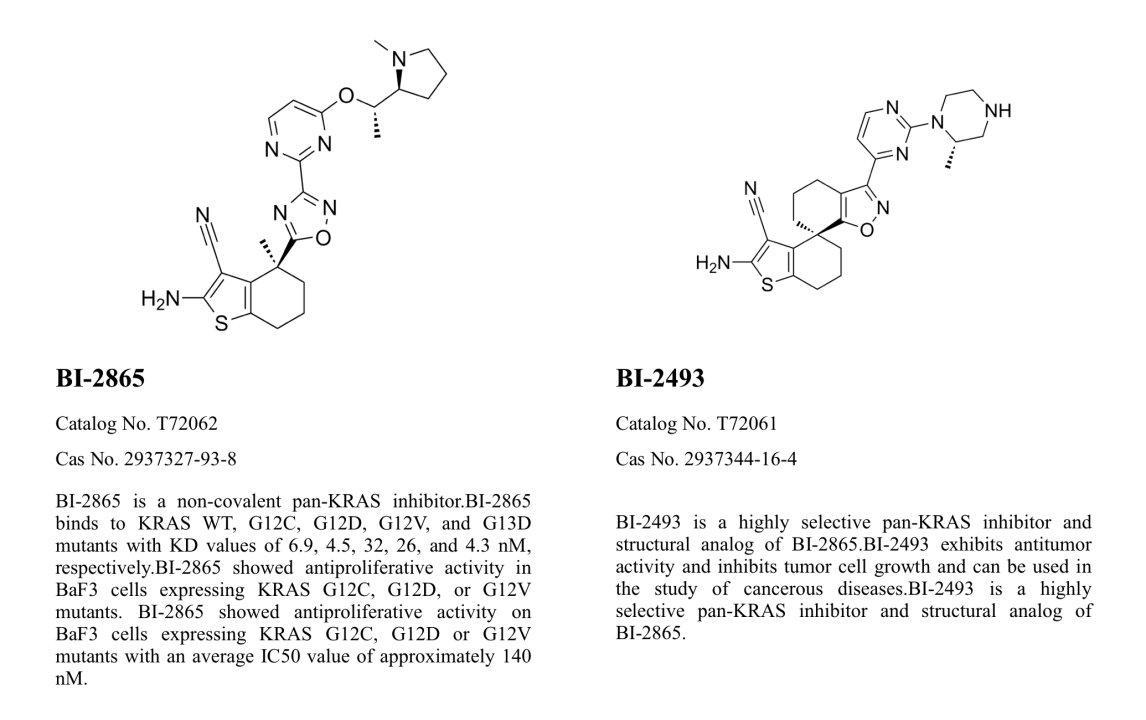
BI-2493, catalog number: T72061, is a highly selective pan-KRAS inhibitor and a structural analogue of BI-2865. It exhibits similar anti-tumor activity to BI-2865, inhibiting tumor cell growth, and is used in research related to cancer diseases.
About KRAS
In common human cancer-causing genes, mutant RAS affects approximately 19% of tumors, making it one of the most prevalent oncogenic mutated genes in human cancers.
Among them, HRAS was first identified in 1982 as an oncogene resulting from a point mutation. Subsequently, NRAS and KRAS were also rapidly discovered. Since then, significant efforts have been made in the study of RAS. Among the three mutations in NRAS, HRAS, and KRAS, KRAS mutations are more common, frequently occurring in pancreatic ductal adenocarcinoma (PDAC), colorectal adenocarcinoma, and lung adenocarcinoma (LUAD), among others.
Mutant RAS proteins exhibit significant oncogenic effects, and tumor cells carrying mutant RAS demonstrate a more invasive phenotype. Patients with tumors harboring RAS mutations generally have a worse prognosis and shorter overall survival compared to those without RAS mutations. Therefore, the development of anti-tumor drugs targeting mutant RAS is imperative.
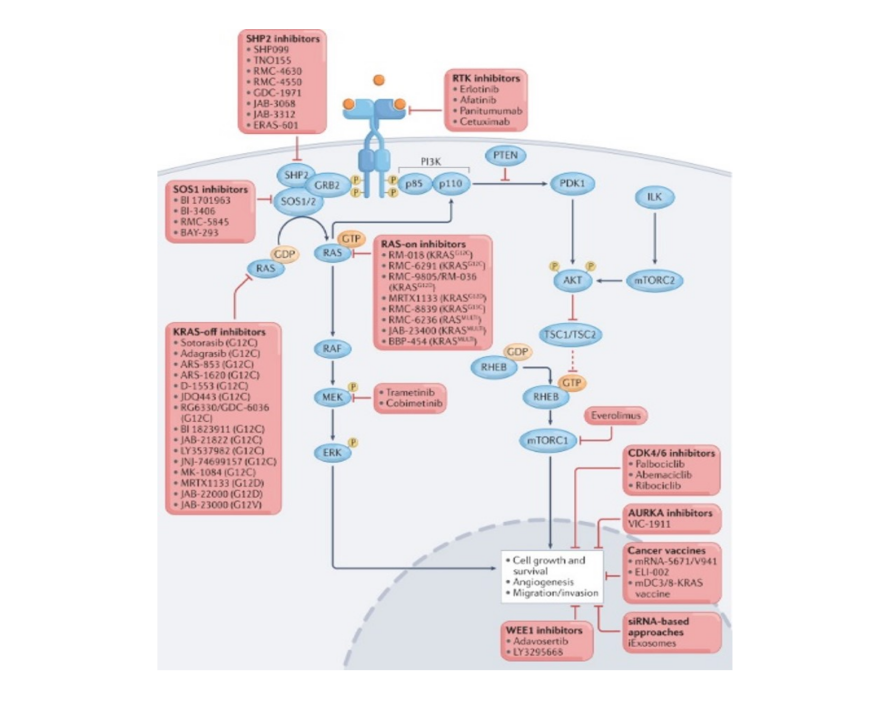
About KRAS inhibitors
(1) KRAS-Specific Inhibitors
KRAS inhibitors can bind to the KRAS protein, rendering it in an inactive state. As mentioned earlier, KRASG12C mutant inhibitors (sotorasib & Adagrasib) irreversibly bind to KRAS, marking a pivotal milestone in clinical drug discovery. However, for another common mutation, KRASG12D (found in 33% of KRAS mutant tumors), there are currently no clinical drugs. Several inhibitors targeting KRASG12D are still in the development stage, with MRTX1133 (Catalog No. T9303) being a prominent candidate.
Preclinical research results demonstrate the specific inhibitory activity of MRTX1133 in various tumor cell lines carrying the KRASG12D mutation. The drug has received FDA approval for a new drug clinical trial application and entered Phase I/II clinical trials in 2023. Although there is considerable research on KRAS mutations, an effective and widely applicable clinical drug has yet to be found. Hence, new methods are needed to identify a class of novel selective KRAS inhibitors.
(2) Pan-KRAS Inhibitors
More than 100 mutation sites have been identified in RAS subtypes, with prominent mutation hotspots at G12, G13, and Q61. However, KRAS-specific inhibitor targets only G12C or G12D mutants, limiting clinical applications. To address these limitations, research teams have begun developing small molecule pan-KRAS inhibitors.
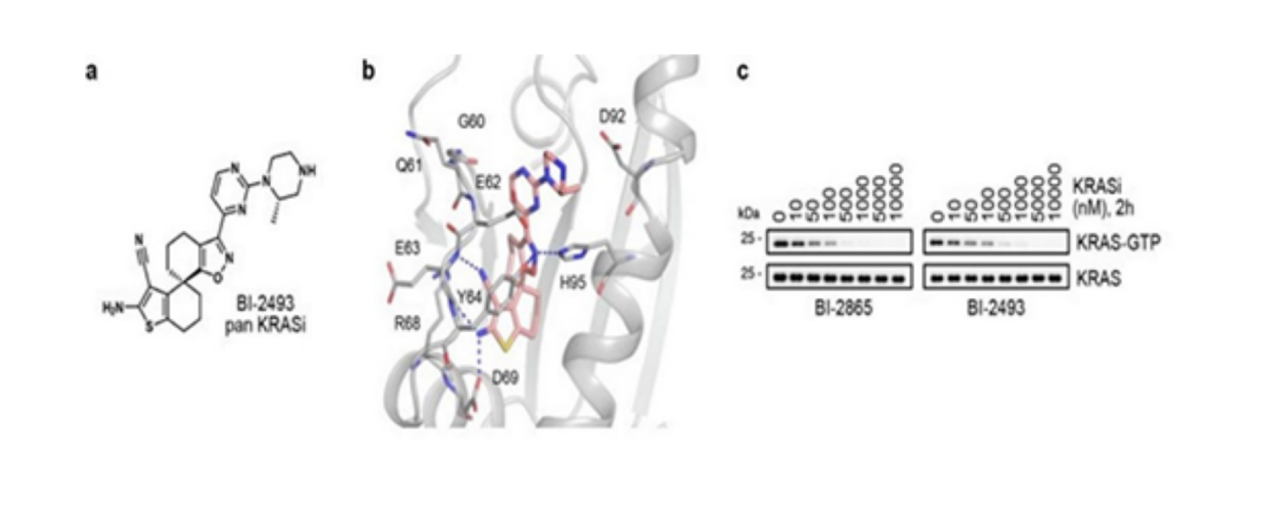
Pan-KRAS inhibitors deactivate common KRAS cancer proteins without the need for covalent anchoring to specific mutant amino acids. These inhibitors can prevent reactivation through nucleotide exchange by preferentially targeting the non-active state of KRAS. Representative examples include BI-2865 and its analogue BI-2493.
Reference
[1] Liu J, Kang R, Tang D. The KRAS-G12C inhibitor: activity and resistance [published correction appears in Cancer Gene Ther. 2023 Dec;30(12):1715]. Cancer Gene Ther. 2022;29(7):875-878. doi:10.1038/s41417-021-00383-9
[2] Nakajima EC, Drezner N, Li X, et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res. 2022;28(8):1482-1486. doi:10.1158/1078-0432.CCR-21-3074
[3] Parada LF, Tabin CJ, Shih C, Weinberg RA. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297(5866):474-478. doi:10.1038/297474a0
[4] Malumbres M, Barbacid M. RAS oncogenes: the first 30 years [published correction appears in Nat Rev Cancer. 2003 Sep;3(9):708]. Nat Rev Cancer. 2003;3(6):459-465. doi:10.1038/nrc1097
[5] Chen K, Zhang Y, Qian L, Wang P. Emerging strategies to target RAS signaling in human cancer therapy. J Hematol Oncol. 2021;14(1):116. Published 2021 Jul 23. doi:10.1186/s13045-021-01127-w
[6] Liu J, Kang R, Tang D. The KRAS-G12C inhibitor: activity and resistance [published correction appears in Cancer Gene Ther. 2023 Dec;30(12):1715]. Cancer Gene Ther. 2022;29(7):875-878. doi:10.1038/s41417-021-00383-9
[7] Wang X, Allen S, Blake JF, et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRASG12D Inhibitor. J Med Chem. 2022;65(4):3123-3133. doi:10.1021/acs.jmedchem.1c01688
[8] Punekar SR, Velcheti V, Neel BG, Wong KK. The current state of the art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol. 2022;19(10):637-655. doi:10.1038/s41571-022-00671-9
[9] Kim D, Herdeis L, Rudolph D, Zhao Y, B?ttcher J, Vides A, Ayala-Santos CI, Pourfarjam Y, Cuevas-Navarro A, Xue JY, Mantoulidis A, Br?ker J, Wunberg T, Schaaf O, Popow J, Wolkerstorfer B, Kropatsch KG, Qu R, de Stanchina E, Sang B, Li C, McConnell DB, Kraut N, Lito P. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023 Jul;619(7968):160-166. doi: 10.1038/s41586-023-06123-3.doi:10.14814/phy2.15092
[10] Kadowaki D, Sumikawa S, Arimizu K, et al. Effect of acetaminophen on the progression of renal damage in adenine induced renal failure model rats. Life Sci. 2012;91(25-26):1304-1308. doi:10.1016/j.lfs.2012.09.018
[11] Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK. Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology. 2013;98(2):116-27. doi: 10.1159/000350435.
Other Articles


Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.