High-Standard Entry Criteria
TargetMol’s FDA-Approved Drug Library is curated with stringent entry criteria to ensure that every compound in the collection is structurally well-defined and of high purity. Multiple analytical techniques—such as NMR, HPLC, and LCMS—are employed to verify compound integrity. Through a rigorous multi-step screening process, we exclude compounds with ambiguous structures (e.g., mixtures and polymers) and non-active agents such as sunscreens, contrast agents, and inorganic compounds. This helps reduce ineffective screening efforts and unnecessary resource consumption.
This library includes only FDA-approved small molecule drugs. For compounds approved by other regulatory agencies (such as PMDA, EMA, and NMPA), please refer to our L1000 Approved Drug Library,L1020 EMA Approved Drug Library
L1000 Approved Drug Library and L1020 EMA Approved Drug Library.
Significant Structural Diversity
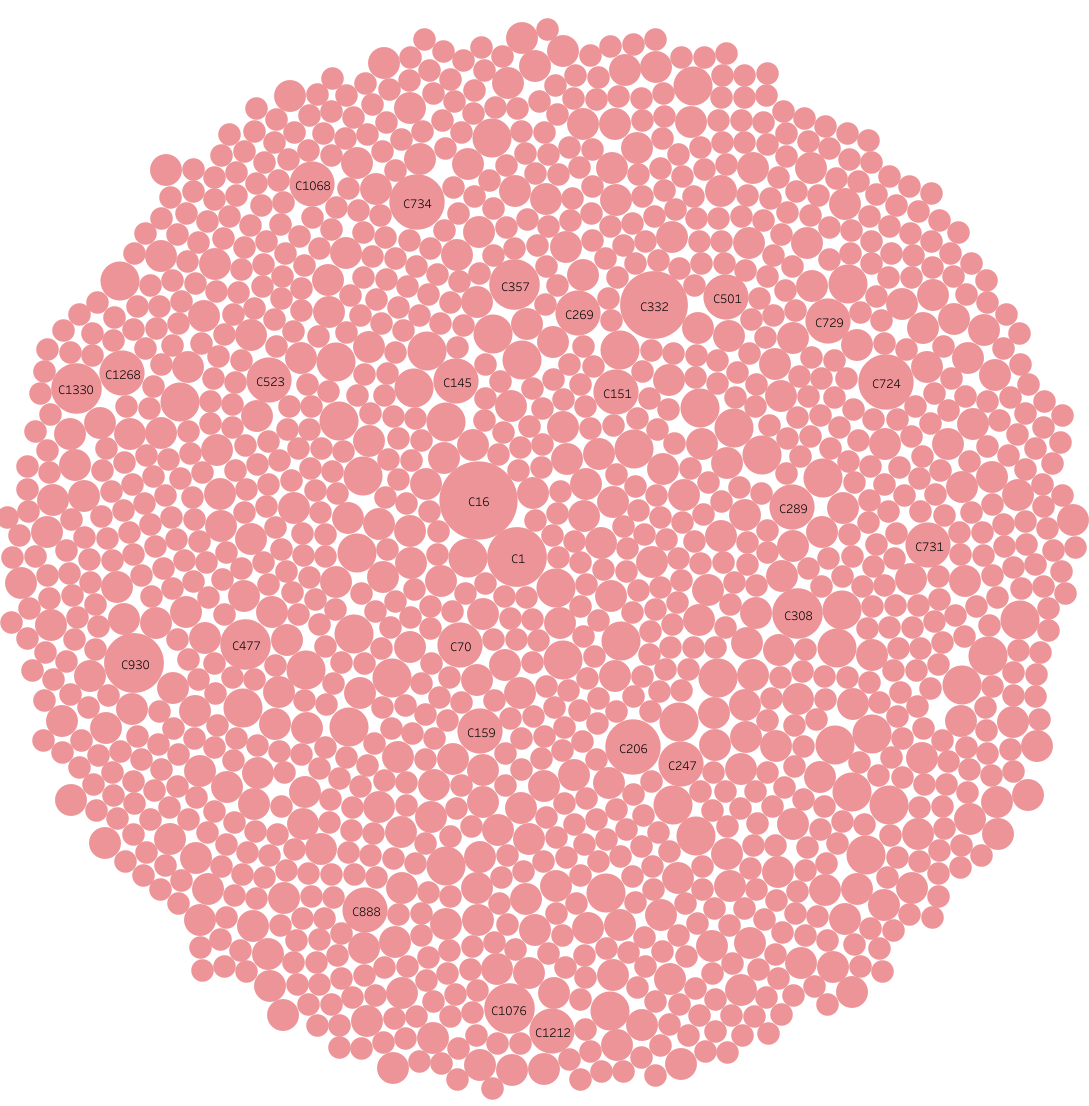
TargetMol’s FDA-Approved Drug Library exhibits remarkable structural diversity, offering substantial advantages in drug discovery. Based on MACCS fingerprint analysis, the library can be categorized into 1,392 classes, effectively covering a broad chemical space. The library includes a wide variety of compounds, ranging from simple to highly complex chemical structures. This diversity provides a wealth of possibilities for identifying lead compounds with high affinity and specificity for target proteins, significantly advancing drug innovation.
Library Diversity Analysis
Diverse Compound Selection
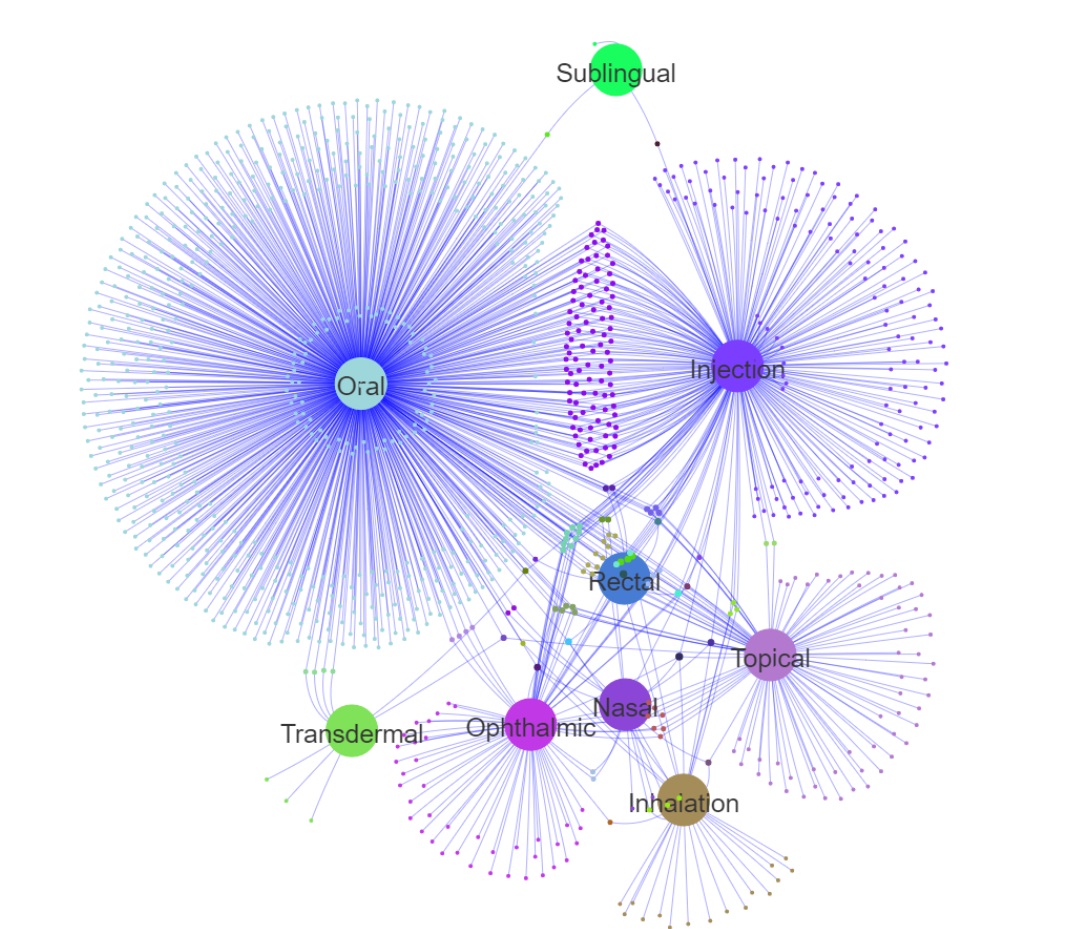
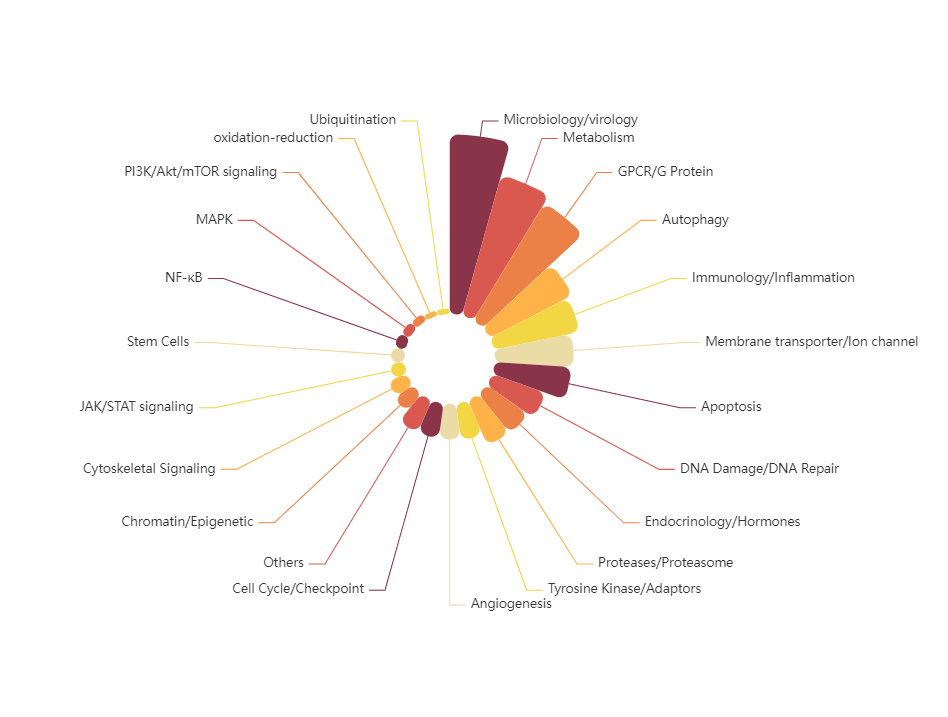
TargetMol's FDA-Approved Drug Library features a highly diverse collection of compounds, both in terms of chemical structure and biological function. The library includes a broad range of key signaling pathway modulators, such as kinase inhibitors, G protein-coupled receptor (GPCR) ligands, ion channel modulators, and epigenetic regulators. These compounds cover a wide array of disease areas including cancer, immunological disorders, neurological diseases, metabolic conditions, and infectious diseases. In addition, the FDA-approved drugs span multiple administration routes, including but not limited to oral, injectable, inhalation, and topical applications. This comprehensive chemical space enables researchers to explore novel therapeutic approaches and accelerate the discovery of innovative medicines.
Drug and Administration Route
Pathways Composition
Disease Types
Regular Updates to Compound Libraries
TargetMol ensures compound libraries stay at the forefront of science by regularly updating our database to include the latest approved and marketed drugs.
Flexible Packaging Options
TargetMol provides a variety of standard packaging sizes (such as 30 μL, 50 μL, 100 μL, 250 μL, and 1 mg), and offer customized packaging solutions tailored to specific needs.
Personalized Custom Services
TargetMol offers fully customized screening services, including the design and synthesis of compound libraries. Our highly flexible service is designed to efficiently meet the unique needs of scientists and researchers.
 Your shopping cart is currently empty
Your shopping cart is currently empty