Your shopping cart is currently empty
Your shopping cart is currently empty
Article | 19 Jan 2024
WIKIMOLE—Sotorasib & Adagrasib
By TargetMol
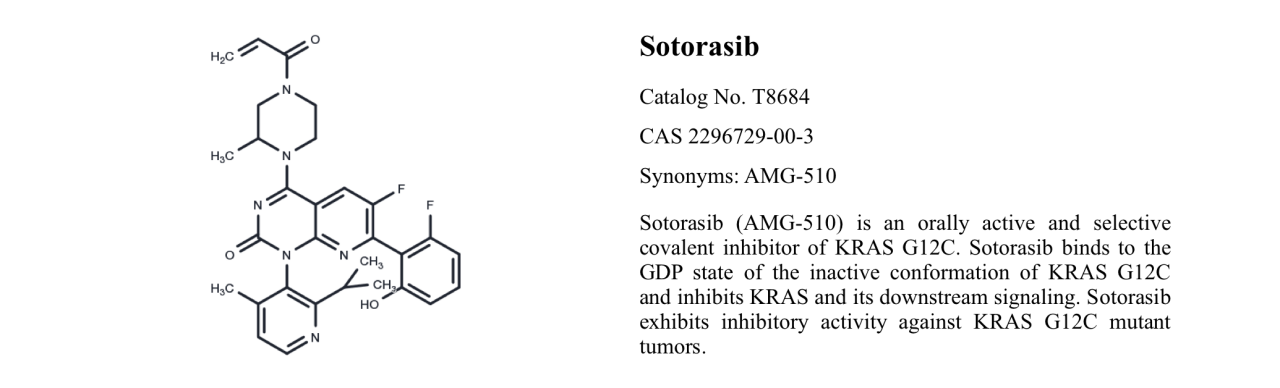
Sotorasib T8684
Sotorasib, also known as AMG-510, is an orally available selective KRASG12C covalent inhibitor. It locks KRASG12C in an inactive GDP-bound state, leading to the regression of KRASG12C-driven tumors.
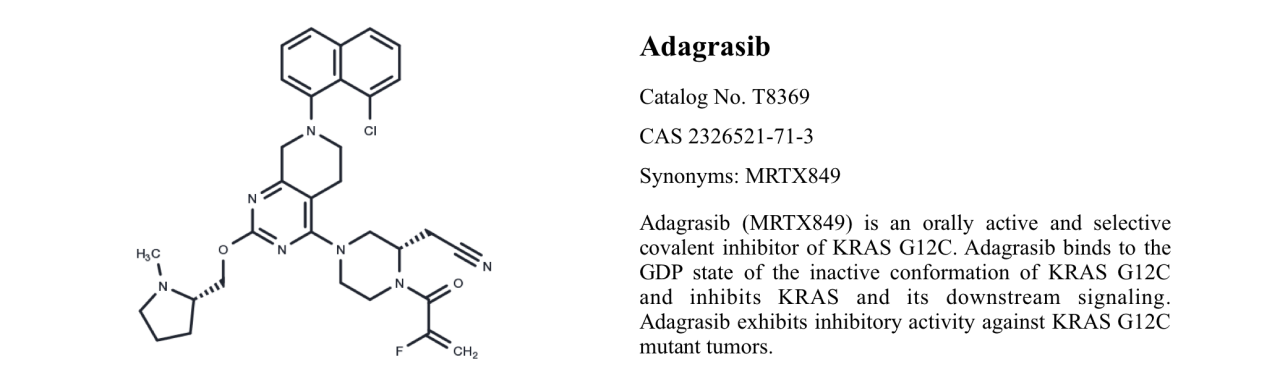
Adagrasib T8369
Adagrasib, also known as MRTX849, is an orally available, mutation-selective KRASG12C covalent inhibitor with potential anti-tumor activity. It locks KRASG12C in an inactive GDP-bound conformation, inhibiting KRAS-dependent signaling transduction.
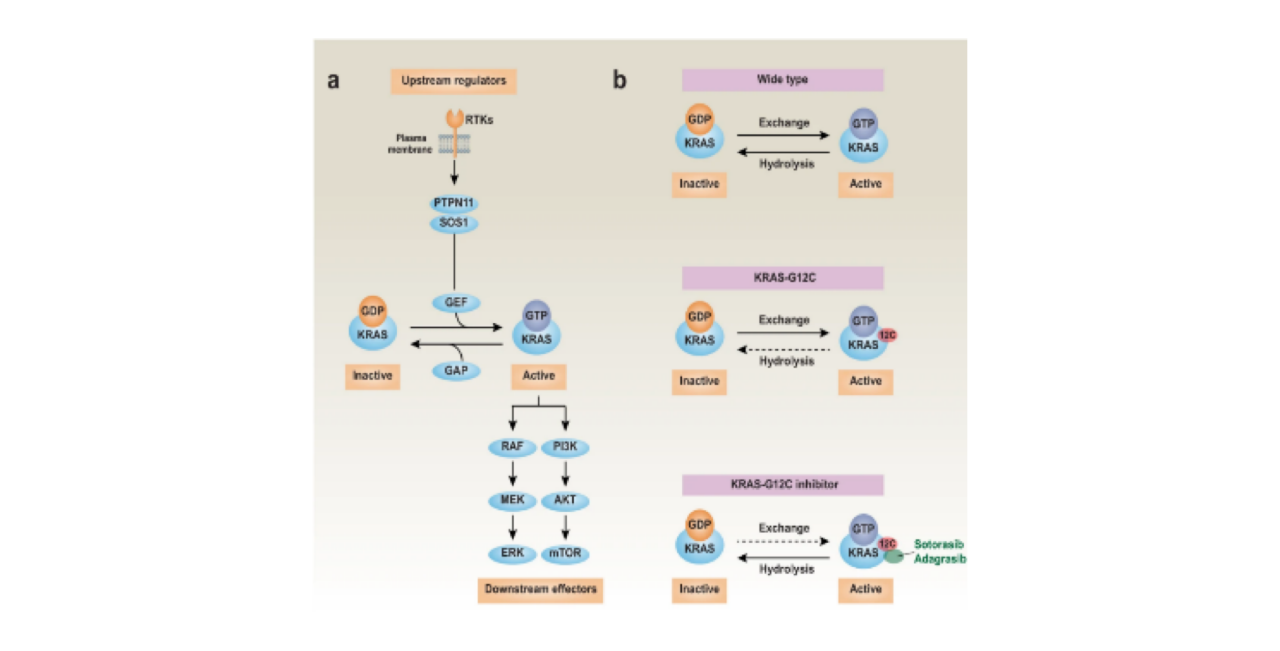
Mechanism of Action
Sotorasib and Adagrasib are selective KRASG12C inhibitors. The KRAS protein is a signaling GTPase that can switch between an active GTP-bound conformation and an inactive GDP-bound conformation. KRASG12C is a specific submutation of KRAS, where glycine at the 12th codon is replaced by cysteine. Both Sotorasib and Adagrasib covalently bind to the cysteine residue in the KRASG12C mutant, locking the KRAS protein in an inactive state. This disruption of downstream signaling pathways contributes to the anti-tumor effects, without affecting the wild-type KRAS.
KRAS can hydrolyze the γ-phosphate of GTP to convert it to GDP. The inactive and active states of KRAS are regulated by GAP and GEF, respectively. RTKs (Receptor Tyrosine Kinases) represent the second-largest class of cell surface receptors with diverse functions, including the promotion of KRAS activation and subsequent involvement in various downstream pathways, notably the RAF-MEK-ERK and PI3K-AKT-mTOR pathways. Unlike wild-type KRAS, which maintains a balance between inactive and activated states, the cysteine 12 (C12) mutation disrupts the GTPase activity of KRAS, locking it in the GTP-bound state.
In contrast, small molecule drugs like sotorasib (or adagrasib) can form a covalent bond with the C12 residue in the KRAS-G12C protein, resulting in KRAS being in an inactive state.
Applications
Sotorasib is the first FDA-approved inhibitor for the treatment of KRASG12C-mutated non-small cell lung cancer (NSCLC). Subsequently, the FDA granted Breakthrough Therapy designation to Adagrasib, providing a potential treatment option for patients with KRASG12C-mutated NSCLC who have previously undergone systemic therapy.
KRASG12C is a specific submutation of KRAS, constituting approximately 44% of all KRAS mutations. It is most commonly found in adenocarcinomas of non-small cell lung cancer, followed by colorectal adenocarcinomas and pancreatic cancers. Globally, over 100,000 people are diagnosed with KRASG12C mutations annually. Sotorasib represents a novel discovery in cancer treatment. Preclinical and clinical studies indicate that cellular differentiation, molecular activation, and genetic mutations can contribute to intrinsic or acquired resistance to KRASG12C inhibitors, including ARS-1620, sotorasib, and adagrasib.
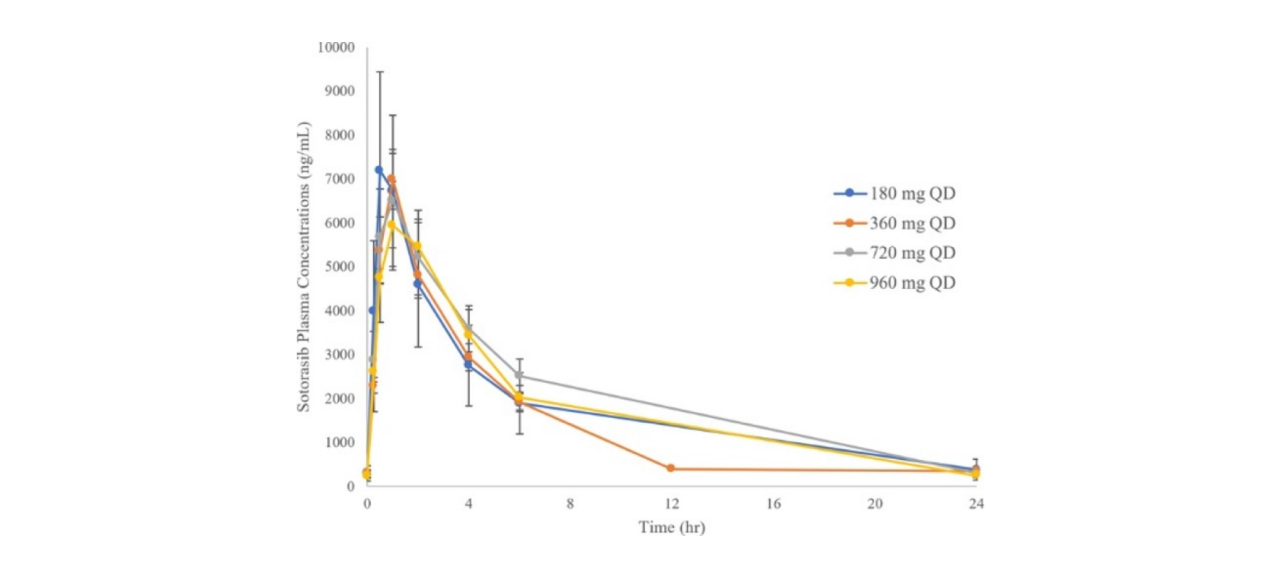
Reference
[1] Liu J, Kang R, Tang D. The KRAS-G12C inhibitor: activity and resistance [published correction appears in Cancer Gene Ther. 2023 Dec;30(12):1715]. Cancer Gene Ther. 2022;29(7):875-878. doi:10.1038/s41417-021-00383-9
[2] Nakajima EC, Drezner N, Li X, et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res. 2022;28(8):1482-1486. doi:10.1158/1078-0432.CCR-21-3074
[3] Chan CH, Chiou LW, Lee TY, et al. PAK and PI3K pathway activation confers resistance to KRASG12C inhibitor sotorasib. Br J Cancer. 2023;128(1):148-159. doi:10.1038/s41416-022-02032-w
[4] Chiou LW, Chan CH, Jhuang YL, Yang CY, Jeng YM. DNA replication stress and mitotic catastrophe mediate sotorasib addiction in KRASG12C-mutant cancer. J Biomed Sci. 2023;30(1):50. Published 2023 Jun 29. doi:10.1186/s12929-023-00940-4
Other Articles


Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.