Your shopping cart is currently empty
Your shopping cart is currently empty
NEWS | 26 July 2024
WIKIMOLE—Sorafenib
By TargetMol
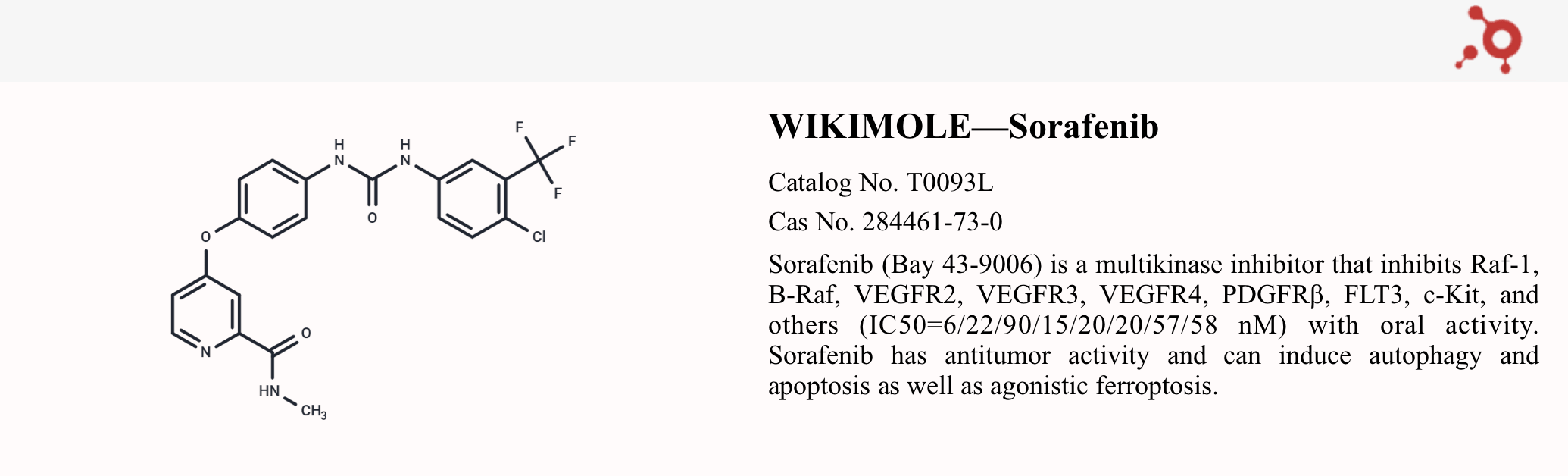
Sorafenib (Cat No. T0093L, CAS 284461-73-0), also known as Bay 43-9006, is an orally active multi-kinase inhibitor. It targets several tumor-related kinases including CRAF, BRAF, c-Kit, FLT-3, VEGFR-2, VEGFR-3, and PDGFR-β.
Mechanism of Action
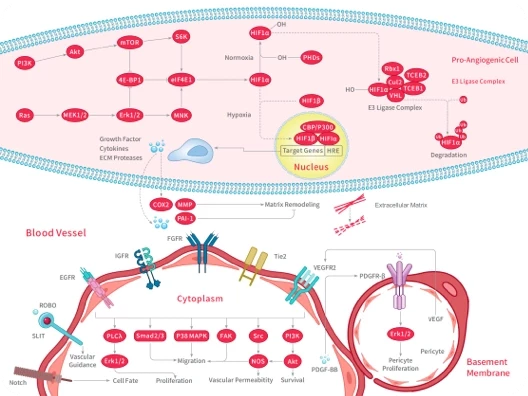
Sorafenib possesses multiple antitumor effects. On one hand, it can directly inhibit tumor growth by blocking the RAF/MEK/ERK signaling pathway. On the other hand, it can indirectly inhibit tumor cell growth by blocking the formation of new tumor blood vessels through inhibition of FGFR, VEGFR, and PDGFR. Additionally, it can induce ferroptosis within cancer cells, leading to their death.
Application
Hepatocellular carcinoma (HCC) has become the fourth leading cause of cancer-related deaths worldwide due to its high mortality rate and high postoperative recurrence rate. Sorafenib is the first molecular targeted drug used for systemic antitumor therapy in liver cancer. Multiple clinical studies have shown that sorafenib provides survival benefits for advanced liver cancer patients across different countries and regions, and with various liver disease backgrounds. The U.S. FDA has approved sorafenib for the treatment of advanced renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and metastatic differentiated thyroid carcinoma (DTC).
Adverse Reactions:
Multiple clinical studies have indicated that common adverse reactions of sorafenib include diarrhea, fatigue, hand-foot syndrome, hypertension, rash, and vomiting.
New Indications:
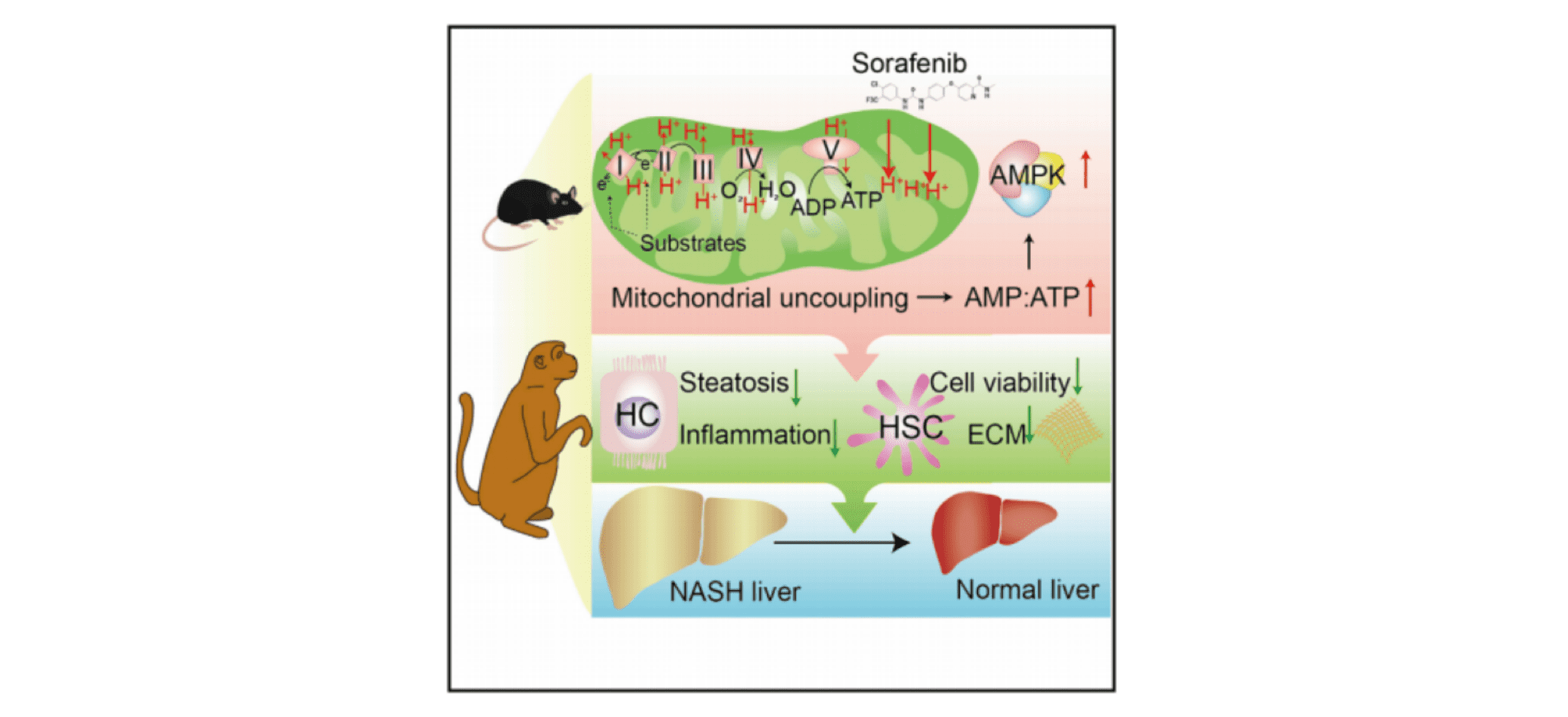
1. At approximately one-tenth of the clinical dose equivalent for HCC, sorafenib effectively halted the progression of NASH in mice and monkeys without observing any significant adverse events.
2. Sorafenib-induced ferroptosis offers a new strategy for treating patients with advanced non-small cell lung cancer (NSCLC) by downregulating MCL1, thereby making NSCLC cells susceptible to ferroptosis.
3. Targeting IL-12-induced Th1 cell differentiation appears to be an effective approach for blocking the development of type 1 diabetes. Through a cell model screen of FDA-approved tyrosine kinase inhibitor (TKI) drug libraries, sorafenib was identified as the preferred inhibitor of Th1 differentiation, suggesting that sorafenib is a potential therapy for human type 1 diabetes.
References
[1]Li Q, Chen K, Zhang T, et al. Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur J Pharmacol. 2023;955:175913. doi:10.1016/j.ejphar.2023.175913
[2]Wei S, Wei F, Li M, et al. Target immune components to circumvent sorafenib resistance in hepatocellular carcinoma. Biomed Pharmacother. 2023;163:114798. doi:10.1016/j.biopha.2023.114798
[3]Yao J, Man S, Dong H, Yang L, Ma L, Gao W. Combinatorial treatment of Rhizoma Paridis saponins and sorafenib overcomes the intolerance of sorafenib. J Steroid Biochem Mol Biol. 2018;183:159-166. doi:10.1016/j.jsbmb.2018.06.010
[4]Li Q, Chen K, Zhang T, et al. Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur J Pharmacol. 2023;955:175913. doi:10.1016/j.ejphar.2023.175913
[5]Zeng Q, Song J, Wang D, et al. Identification of Sorafenib as a Treatment for Type 1 Diabetes. Front Immunol. 2022;13:740805. Published 2022 Feb 15. doi:10.3389/fimmu.2022.740805
Other Articles


Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.