Your shopping cart is currently empty
Your shopping cart is currently empty
Article | 02 Feb 2024
WIKIMOLE—Orforglipron
By TargetMol

GLP-1RA and SGLT-2i are new diabetes medications that have gained widespread attention due to their cardiovascular and renal benefits brought to patients with T2D.
Orforglipron (Catalog No. T11408), also known as LY3502970, is a GLP-1 receptor agonist. It is used in research related to obesity and type 2 diabetes (T2D).
Preclinical Research
Orforglipron belongs to a new class of chemically synthesized oral non-peptide drugs that exhibit effective anti-diabetic effects by enhancing glucose-dependent insulin secretion and improving energy balance. In preclinical models, orforglipron has shown promising efficacy in lowering elevated blood glucose levels in experimental animals and exhibits favorable pharmacokinetic characteristics for oral administration.
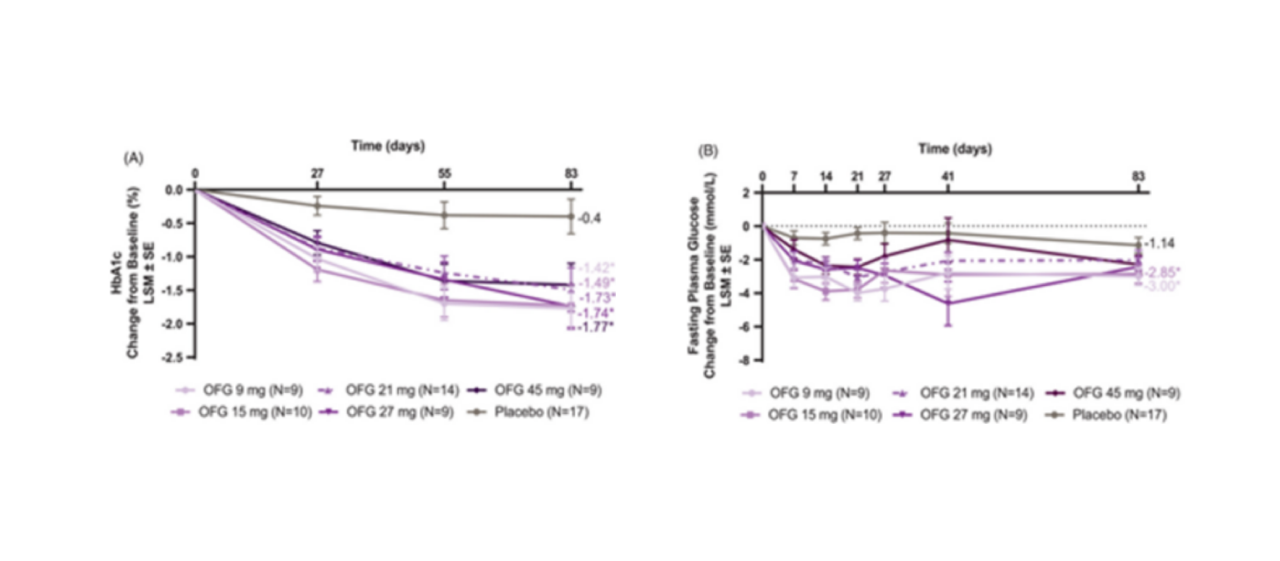
Currently, Orforglipron, as a medication for improving blood sugar control in adults and managing chronic body weight (weight loss) in adults, initiated Phase 3 clinical trials in September 2023. Results from the Phase 1 clinical study of Orforglipron indicate its GLP-1-like effects, making it a potential treatment for obesity and type 2 diabetes, comparable to other GLP-1 analogs. Phase 2 clinical data suggests that Orforglipron treatment significantly reduces blood sugar and body weight, without any clinically inconsistent adverse events compared to other GLP-1 receptor agonists. Its pharmacokinetic profile allows for once-daily oral administration, without dietary restrictions on food or water, providing a potentially safe and effective oral treatment option for patients with type 2 diabetes (T2D) and other indications. The current mainstream method of administration is subcutaneous injection; however, oral medications offer greater convenience and accessibility, eliminating the fear of injections for some patients.
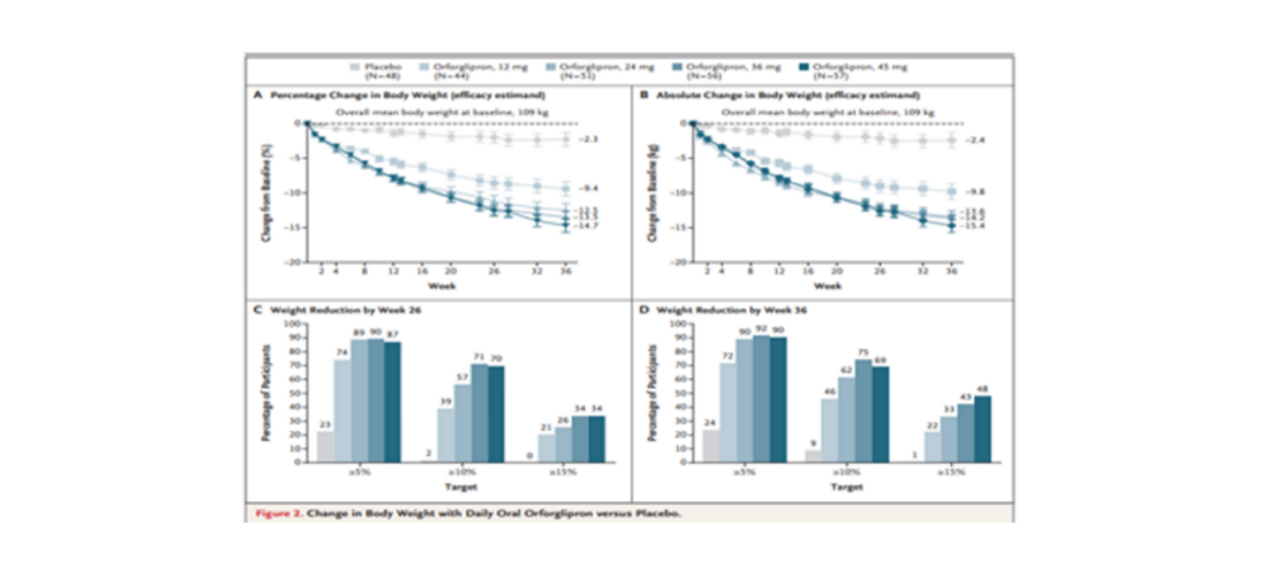
The latest data from Phase 2 clinical trials show that different doses of Orforglipron, when taken orally, can significantly reduce patient weight by up to approximately 13% at 26 weeks and around 15% at 36 weeks. This study not only sets the stage for future Phase 3 weight loss clinical trials but also rejuvenates the weight loss market. The Phase 3 clinical trial plans to enroll 1576 patients with type 2 diabetes and is expected to be completed by July 2025.
Orforglipron vs Semaglutide
Semaglutide (Catalog No. T19850), also known as Semaglutide, is an FDA-approved medication and a GLP-1 receptor agonist. Currently, it is primarily used for treating type 2 diabetes (T2D) and adverse events in T2D patients with cardiovascular diseases. It is also employed for treating adult obesity, although its indication for obesity in China has not yet been approved. The drug supports both injection and oral administration. However, its oral bioavailability is low, and patients are required to adhere to strict dietary restrictions during medication. Fasting is necessary before taking the medication. This is an aspect where Orforglipron excels over Semaglutide, as the former offers better patient compliance with oral administration.
Reference
[1] Kawai T, Sun B, Yoshino H, Feng D, Suzuki Y, Fukazawa M, Nagao S, Wainscott DB, Showalter AD, Droz BA, Kobilka TS, Coghlan MP, Willard FS, Kawabe Y, Kobilka BK, Sloop KW. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29959-29967. doi: 10.1073/pnas.2014879117. Epub 2020 Nov 11. PMID: 33177239; PMCID: PMC7703558.
[2] Pratt E, Ma X, Liu R, et al. Orforglipron (LY3502970), a novel, oral non-peptide glucagon-like peptide-1 receptor agonist: A Phase 1b, multicentre, blinded, placebo-controlled, randomized, multiple-ascending-dose study in people with type 2 diabetes. Diabetes Obes Metab. 2023;25(9):2642-2649. doi:10.1111/dom.1515
[3] Wharton S, Blevins T, Connery L, et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N Engl J Med. 2023;389(10):877-888. doi:10.1056/NEJMoa2302392
Other Articles


Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.