 Your shopping cart is currently empty
Your shopping cart is currently empty

Dexamethasone is a glucocorticoid receptor agonist and IL receptor modulator with anti-inflammatory, immunosuppressive, and apoptosis-inducing activities. It inhibits the production of inflammatory miRNA-155 exosomes in macrophages, significantly reduces inflammatory cytokine expression in neutrophils and monocytes, suppresses LPS-induced macrophage inflammation, and induces autophagy. It is commonly used to induce animal models of depression, muscle atrophy, and hypertension, and holds potential in COVID-19 research.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 50 mg | $29 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $45 | In Stock | In Stock |
| Description | Dexamethasone is a glucocorticoid receptor agonist and IL receptor modulator with anti-inflammatory, immunosuppressive, and apoptosis-inducing activities. It inhibits the production of inflammatory miRNA-155 exosomes in macrophages, significantly reduces inflammatory cytokine expression in neutrophils and monocytes, suppresses LPS-induced macrophage inflammation, and induces autophagy. It is commonly used to induce animal models of depression, muscle atrophy, and hypertension, and holds potential in COVID-19 research. |
| Targets&IC50 | HEK293T cells:> 20 μM, Macrophages:0.134 μM, CHO cells:> 20 μM, BV-2 cells:2.5 x 10-2 μM, B16 F10 cells:> 20 μM, HeLa S3 cells:4.1 nM, A549 cells:> 15 μM, transcription of the β2-receptor:36 nM (EC50), Fibroblast:> 15 μM, B-cell:0.8 μM, granulocyte-Macrophage cells colony stimulating factor:2.2 nM (EC50), J774 cells:5.2 μM, NF-κB, IκBα, and IκBβ:0.5 nM, COS-7 cells:7.2 nM (EC50), HEK293 cells:> 500 μM |
| In vitro | METHODS: Human colorectal cancer cells LoVo and HCT116 were treated with Dexamethasone (10-300 µM) for 72 h. Cell growth inhibition was detected by MTT. RESULTS: Dexamethasone dose-dependently inhibited the growth of LoVo and HCT116 cells, and the inhibition rates of 300 µM Dexamethasone were 52.6% and 58.8%, respectively. [1] METHODS: Acute lymphoblastic leukemia cells RS4;11 were treated with Dexamethasone (100 nM) for 24-36 h. Cell morphology was examined using electron microscopy. RESULTS: In cells treated with Dexamethasone for 24 h, vesicles were surrounded by double membranes, which are characteristic of autophagosomes, and contained membrane structures and/or part of the endoplasmic reticulum or a large amount of cytoplasm. In addition to the appearance of autophagosomes, the nucleus and cell morphology were initially intact, suggesting that autophagosome formation preceded cell death. Dexamethasone induces autophagy. [2] METHODS: Activated mouse and human T cells were treated with Dexamethasone (0.001-10 μM) for 48 h, and PD-1 expression was detected by Flow Cytometry. RESULTS: Dexamethasone enhanced the expression of PD-1 in mouse and human activated T cells. [3] |
| In vivo | METHODS: To investigate the anti-inflammatory effects, Dexamethasone (1-10 mg/kg) was administered as a single intraperitoneal injection to LPS-induced inflammation in C57Bl/6JBom mice. RESULTS: 10 mg/kg Dexamethasone significantly reduced neutrophils in bronchoalveolar lavage fluid.Dexamethasone treatment significantly down-regulated the levels of TNF-α, IL-1α, IL-1β, IL-6, IL-12p40 and MIP-1α mRNA. Dexamethasone exerts anti-inflammatory and antioxidant functions in acute airway inflammation. [4] METHODS: To detect anti-tumor activity in vivo, Dexamethasone (1 mg/kg) was intraperitoneally injected into SCID mice harboring the human myeloma tumor OPM2 five days per week for three weeks. RESULTS: Dexamethasone treatment significantly inhibited the growth of OPM2 tumors, indicating antitumor activity in vivo. [5] |
| Animal Research | NAC was administered at three different doses (10, 100 and 500 mg/kg body weight). At the highest concentration, the acidic pH of the NAC solution was adjusted by adding NaOH. Dexamethasone was administered as a single injection of 1 or 10 mg/kg. Both drugs were dissolved in saline and 400 μl were injected intraperitoneally, either 1 h before or 1 h after LPS exposure. In one experiment, NAC (100 and 500 mg/kg) was injected successively every 4·5 h, starting 1 h before challenge (five injections in total). A control group of LPS-exposed animals were injected intraperitoneally with solvent alone (saline). Intratracheal administration was performed by instillation of 100 μl NAC (50, 100 or 500 mg/kg) or dexamethasone (10 mg/kg) into the lungs of mice anaesthetized with 15 mg/kg Rapinovet (i.v.) [4]. |
| Synonyms | Prednisolone F, NSC 34521, MK 125, Hexadecadrol |
| Molecular Weight | 392.46 |
| Formula | C22H29FO5 |
| Cas No. | 50-02-2 |
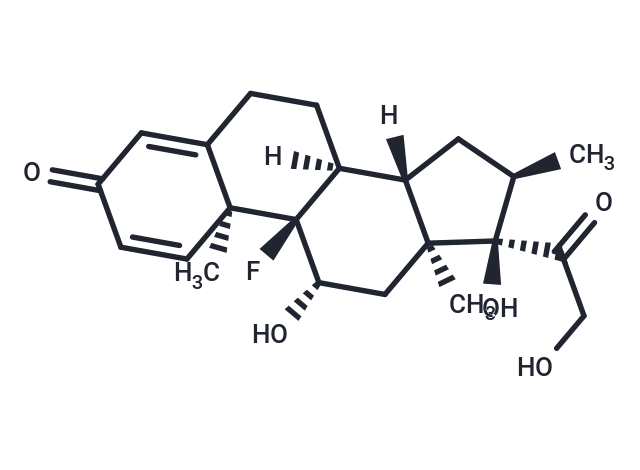
| Smiles | F[C@@]12[C@]([C@]3([C@](C)(C[C@@H]1O)[C@](C(CO)=O)(O)[C@H](C)C3)[H])(CCC=4[C@]2(C)C=CC(=O)C4)[H] |
| Relative Density. | 1.32 g/cm3 |
| Color | White |
| Appearance | Solid |
| Storage | keep away from direct sunlight | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: insoluble Ethanol: 6 mg/mL (15.29 mM), Sonication is recommended. DMSO: 250 mg/mL (637.01 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 7.3 mg/mL (18.6 mM), Suspension. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.