Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
L-Ascorbic acid sodium salt (Vitamin C sodium salt) is a more bioavailable form of vitamin C that is an alternative to taking ascorbic acid as a supplement.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 200 mg | $30 | In Stock | In Stock | |
| 500 mg | $40 | In Stock | In Stock | |
| 1 g | $48 | In Stock | In Stock | |
| 5 g | $113 | - | In Stock | |
| 10 g | $163 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $39 | In Stock | In Stock |
| Description | L-Ascorbic acid sodium salt (Vitamin C sodium salt) is a more bioavailable form of vitamin C that is an alternative to taking ascorbic acid as a supplement. |
| In vitro | Sodium ascorbate has a growth inhibiting action only at high concentrations in cultured human neoplastic cell lines MCF-7 (breast carcinoma), KB (oral epidermoid carcinoma), and AN3-CA (endometrial adenocarcinoma). Sodium ascorbate combined with vitamin K3 demonstrates a synergistic inhibition of cell growth at 10 to 50 times lower concentrations in cultured human neoplastic cell lines MCF-7, KB, and AN3-CA, at this level separately given vitamins are not toxic. This tumor cell growth inhibitory effect is completely suppressed by the addition of catalase to the culture medium containing vitamins C and K3, suggesting an excessive production of hydrogen peroxide as being implied in mechanisms responsible for the above-mentioned effects. [1] Sodium ascorbate combined with vitamin K3 results in a synergistic effect on growth inhibition in cultured human endometrial adenocarcinoma (AN3CA) cells. [2] Sodium ascorbate results in a rapid increase in the intracellular concentration of Ca2+ ions and subsequent apoptotic cell death in HL-60 cells, characterized by cell shrinkage, nuclear fragmentation and cleavage of internucleosomal DNA to yield fragments that are multiples of 180-200 base pairs, are induced. [3] Sodium ascorbate (100 μM) induces DNA single-strand breaks in human cells, Fibroblasts and Molt-4 cells are significantly more sensitive than lymphocytes. Sodium ascorbate (50 μM) results in significant cell loss in Molt-4 cells, but not in lymphocyte and fibroblast cultures. [4] |
| In vivo | Tg rats treated with sodium?L-ascorbate show a higher incidence of carcinoma (29.6%), compared to those without sodium?L-ascorbate (15.4%). Independent of the sodium?L-ascorbate treatment, transgenic rats exhibit various kinds of malignant tumors in various organs[5]. After 12 weeks of PEITC-treatment, both simple hyperplasia and papillary or nodular (PN) hyperplasia have developed in all animals, but the majority of these lesions have disappeared at week 48, irrespective of the sodium?L-ascorbate-treatment. The same lesions after 24 weeks of PEITC-treatment have progressed to dysplasia and carcinoma, in a small number of cases by week 48, but enhancement by the sodium?L-ascorbate-treatment is evident only with simple hyperplasias and PN hyperplasias in rats[6]. |
| Synonyms | Vitamin C sodium salt, Sodium L-ascorbate, Sodium ascorbate, L-Ascorbic acid sodium, (+)-Sodium L-ascorbate |
| Molecular Weight | 198.11 |
| Formula | C6H7NaO6 |
| Cas No. | 134-03-2 |
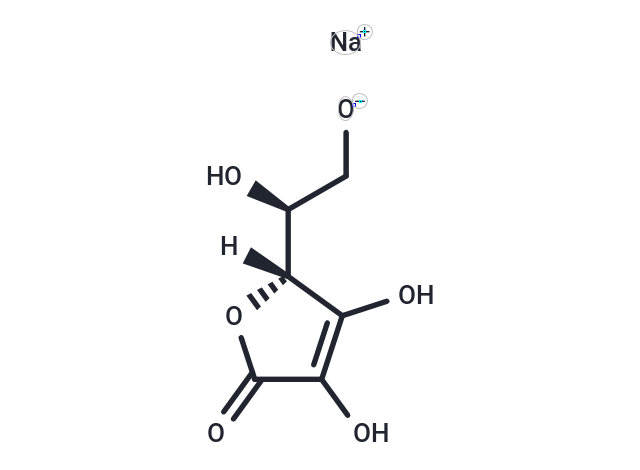
| Smiles | [O-]C[C@@H]([C@]1([H])C(O)=C(C(O1)=O)O)O.[Na+] |
| Relative Density. | 1.88. Temperature:19.7 °C. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 6.25 mg/mL (31.55 mM), Sonication is recommended. H2O: 198.9 mM, Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
DMSO/H2O
H2O
| |||||||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.