 Your shopping cart is currently empty
Your shopping cart is currently empty

Fludarabine (Fludarabinum) is a fluorinated purine analog, an inhibitor of nucleic acid synthesis and an inhibitor of STAT1 activation. Fludarabine has antitumor activity and can be used for the treatment of leukemia and lymphoma.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 mg | $50 | In Stock | In Stock | |
| 10 mg | $52 | In Stock | In Stock | |
| 25 mg | $80 | In Stock | In Stock | |
| 50 mg | $100 | In Stock | In Stock | |
| 100 mg | $141 | In Stock | In Stock | |
| 200 mg | $197 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock | In Stock |
| Description | Fludarabine (Fludarabinum) is a fluorinated purine analog, an inhibitor of nucleic acid synthesis and an inhibitor of STAT1 activation. Fludarabine has antitumor activity and can be used for the treatment of leukemia and lymphoma. |
| Targets&IC50 | RPMI8226 cells:1.54 µg/mL, CCRF-CEM cells:19.49 μM, HepG2 cells:20 μM, K562 cells:0.26 μM, PBMC cells:1.9 μM, HCT116 cells:6.6 μM, MM.1S cells:13.48 µg/mL, A549 cells:47.44 μM, HeLa cells:16 μM, MCF-7 cells:15 μM, T47D cells:46.2 μM, MM.1R cells:33.79 µg/mL, Mahlavu cells:10 μM |
| In vitro | METHODS: Multiple myeloma cells RPMI8226, MM.1S and MM.1R were treated with Fludarabine (0-64 µg/mL) for 24-48 h. Cell viability was measured by MTT Assay. RESULTS: Fludarabine dose-time-dependently inhibited the proliferation of RPMI8226 cells with an IC50 of 1.54 µg/mL at 24 h. At 48 h, the IC50 of Fludarabine on MM.1S and MM.1R cells was 13.48 µg/mL and 33.79 µg/mL, respectively. [1] METHODS: Rat aortic VSMCs were treated with Fludarabine (50 µM) and FBS for 30 min, and the expression levels of target proteins were detected by Western Blot. RESULTS: FBS stimulation produced progressive JAK2 and STAT-1 activation, and Fludarabine induced a significant reduction in STAT-1 phosphorylation, while it did not alter JAK2 activation. [2] |
| In vivo | METHODS: To assay antitumor activity in vivo, Fludarabine (8-40 mg/kg) was injected intraperitoneally into SCID mice bearing multiple myeloma RPMI8226 once daily for three days. RESULTS: The antitumor activity of Fludarabine in vivo was demonstrated by a less than 5-fold increase in tumors treated with 40 mg/kg of Fludarabine over 25 days compared to an approximately 10-fold increase in control tumors. [1] METHODS: To study the effect on graft-versus-host disease (GVHD), Fludarabine (0.8 mg/kg) was administered intraperitoneally to (BALB/c x C57BL/6) F1 mice harboring B-cell leukemia (BCL-1) every two weeks for five days in two cycles, followed by intraperitoneal injection of cyclophosphamide (400 mg/kg). RESULTS: Mice treated with a Fludarabine-containing regimen prior to transplantation also had much less GVHD clinically and at necropsy, while graft-versus-leukemia appeared to be increased in the same animals. [3] |
| Cell Research | VSMCs were isolated from the aorta of male Wistar rats weighing ~350–500 g, as previously described. For cell culture experiments, 2 × 10^5 rat VSMCs were plated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). Semiconfluent VSMCs were starved by incubation in 0.5% FBS/DMEM for 36–48 h and then serum-stimulated with normal growth medium (i.e., DMEM containing 10% FBS) in the presence or absence of fludarabine (50 μM) [2]. |
| Animal Research | The animals in this study were handled according to the animal welfare regulation of the Magna Graecia University of Catanzaro, and the protocol was approved by the animal use committee of this institution. Fifty Wistar rats weighing 340 ± 40 g were anesthetized with an intramuscular injection of 100 mg/kg ketamine and 5 mg/kg xylazine. Angioplasty of the common carotid artery was performed using a balloon embolectomy catheter, as previously described and well validated in our laboratory. Fludarabine was dissolved in 30% pluronic F127 gel to the final concentrations of 2.5, 5, 15, or 25 mg/ml. At the time of balloon injury, gel containing fludarabine or vehicle was applied around the middle segment (2 cm in length) of the right injured carotid artery (0.1 ml per 1-cm length of the artery segment, equivalent to 0.5, 1, 3, or 5 mg of total fludarabine locally delivered), as previously described. As a control experiment, 200 μl of fludarabine/gel solution (25 mg/ml) were applied around the sham-operated carotid artery. To study the fludarabine toxicity, laboratory studies were performed at baseline and 2 wk after drug local delivery (25 mg/ml). Arterial pressure and heart rate were measured indirectly by a tail-cuff plethysmographic technique [2]. |
| Synonyms | NSC 118218, Fludarabinum, F-ara-A |
| Molecular Weight | 285.23 |
| Formula | C10H12FN5O4 |
| Cas No. | 21679-14-1 |
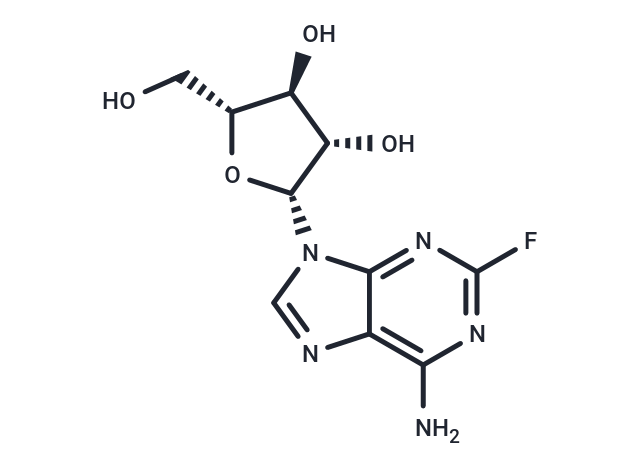
| Smiles | NC1=C2N=CN([C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)C2=NC(F)=N1 |
| Relative Density. | 2.17g/cm3 |
| Storage | store at low temperature,keep away from direct sunlight,keep away from moisture | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 240 mg/mL (841.43 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2.86 mg/mL (10.03 mM), Solution. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.