- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Shopping Cart
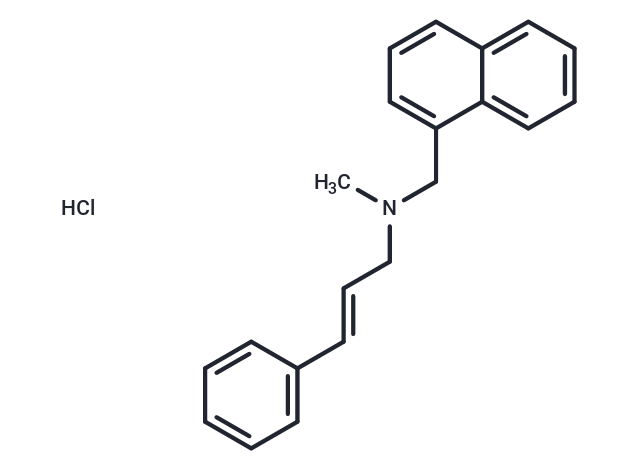
Naftifine hydrochloride
Catalog No. T1543Cas No. 65473-14-5
Alias Naftin, Naftifungin, Naftifine HCl, Exoderil
Naftifine Hydrochloride is the hydrochloride salt form of naftifine, an allylamine derivate with synthetic broad-spectrum antifungal activity. Although the exact mechanism through which naftifine hydrochloride exerts its effect is unknown, it appears to selectively inhibit the enzyme squalene 2, 3-epoxidase, thereby inhibiting the biosynthesis of sterol. This results in a decreased amount of sterols, especially ergosterol which is the primary fungal membrane sterol, and a corresponding accumulation of squalene in fungal cells. Naftifine hydrochloride (Naftifine HCl) can be fungicidal as well as fungistatic to yeasts depending on the concentration and the organisms involved.
Naftifine hydrochloride
Catalog No. T1543Alias Naftin, Naftifungin, Naftifine HCl, ExoderilCas No. 65473-14-5
Naftifine Hydrochloride is the hydrochloride salt form of naftifine, an allylamine derivate with synthetic broad-spectrum antifungal activity. Although the exact mechanism through which naftifine hydrochloride exerts its effect is unknown, it appears to selectively inhibit the enzyme squalene 2, 3-epoxidase, thereby inhibiting the biosynthesis of sterol. This results in a decreased amount of sterols, especially ergosterol which is the primary fungal membrane sterol, and a corresponding accumulation of squalene in fungal cells. Naftifine hydrochloride (Naftifine HCl) can be fungicidal as well as fungistatic to yeasts depending on the concentration and the organisms involved.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 mg | $35 | In Stock | |
| 500 mg | $84 | In Stock | |
| 1 g | $109 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $30 | In Stock |
Add to Cart
Add to Quotation
Bulk & Custom
Questions
View MoreSelect Batch
Purity:99.84%
Contact us for more batch information
All TargetMol products are for research purposes only and cannot be used for human consumption. We do not provide products or services to individuals. Please comply with the intended use and do not use TargetMol products for any other purpose.Product Introduction
Bioactivity
Chemical Properties
| Description | Naftifine Hydrochloride is the hydrochloride salt form of naftifine, an allylamine derivate with synthetic broad-spectrum antifungal activity. Although the exact mechanism through which naftifine hydrochloride exerts its effect is unknown, it appears to selectively inhibit the enzyme squalene 2, 3-epoxidase, thereby inhibiting the biosynthesis of sterol. This results in a decreased amount of sterols, especially ergosterol which is the primary fungal membrane sterol, and a corresponding accumulation of squalene in fungal cells. Naftifine hydrochloride (Naftifine HCl) can be fungicidal as well as fungistatic to yeasts depending on the concentration and the organisms involved. |
| In vitro | Naftifine demonstrates a broad in vitro activity spectrum against dermatophytes (38 strains; MIC 0.1 to 0.2 mg/mL), aspergilli (6 strains; MIC 0.8 to 12.5 mg/mL), Sporothrix schenckii (2 strains; MIC 0.8 and 1.5 mg/mL), and Candida yeasts (77 strains; MIC 1.5 to >100 mg/mL). [1] For C. albicans Δ63, the MIC is 100 mg/L in Sabouraud medium (initial pH 6.5). At 50 mg/L, naftifine inhibits sterol biosynthesis by over 99% in both whole cells and cell extracts of C. albicans. Its primary mode of action is blocking fungal squalene epoxidation. [2] |
| In vivo | Naftifine HCl 2% cream results in clinical cure rate and clinical success rate of 33% and 84% after treatment for 4 weeks, and week 2 efficacy response rates in Naftifine HCl 2% subjects are all lower than at week 4 but are significantly higher than week 2 vehicle-treated counterparts. [3] Naftifine causes interruption of fungal ergosterol synthesis and accumulation of squalene in fungal organisms. Naftifine also has demonstrated anti-inflammatory properties such as a reduction in superoxide production and a reduction in polymorphonuclear leukocyte chemotaxis/endothelial adhesion. Naftifine has shown good efficacy and safety for a variety of conditions and is a useful treatment that provides both antifungal action and relief of inflammatory signs and symptoms. Few adverse events have been noted with naftifine use, the most frequent being mild and transient burning, stinging, or itching in the application area. [4] |
| Alias | Naftin, Naftifungin, Naftifine HCl, Exoderil |
| Molecular Weight | 323.86 |
| Formula | C21H22ClN |
| Cas No. | 65473-14-5 |
| Smiles | Cl.CN(C\C=C\c1ccccc1)Cc1cccc2ccccc12 |
| Relative Density. | no data available |
Storage & Solubility Information
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 100 mg/mL (308.77 mM), Sonication is recommended. H2O: 2.5 mg/mL (7.72 mM), Sonication is recommended. Ethanol: 8 mg/mL (24.7 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||||||||||||
H2O/Ethanol/DMSO
Ethanol/DMSO
DMSO
| ||||||||||||||||||||||||||||||||||||||||||||||
Calculator
In Vivo Formulation Calculator (Clear solution)
Please enter your animal experiment information in the following box and click Calculate to obtain the mother liquor preparation method and in vivo formula preparation method:
Mother liquor preparation method: 2 mg of drug dissolved in 50 μL DMSO (mother liquor concentration of 40 mg/mL), if you need to configure a concentration that exceeds the solubility of the product, please contact us first.
(mother liquor concentration of 40 mg/mL), if you need to configure a concentration that exceeds the solubility of the product, please contact us first.
Preparation method for in vivo formula: Take 50 μL DMSO main solution, add 300 μLPEG300
main solution, add 300 μLPEG300 mix well and clarify, then add 50 more μL Tween 80, mix well and clarify, then add 600 more μLSaline/PBS/ddH2O
mix well and clarify, then add 50 more μL Tween 80, mix well and clarify, then add 600 more μLSaline/PBS/ddH2O mix well and clarify
mix well and clarify
For Reference Only. Please develop an appropriate dissolution method based on your laboratory animals and route of administration.
Dose Conversion
You can also refer to dose conversion for different animals. More Dose Conversion
Sci Citations
Tech Support
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc
Keywords
Related Tags: buy Naftifine hydrochloride | purchase Naftifine hydrochloride | Naftifine hydrochloride cost | order Naftifine hydrochloride | Naftifine hydrochloride chemical structure | Naftifine hydrochloride in vivo | Naftifine hydrochloride in vitro | Naftifine hydrochloride formula | Naftifine hydrochloride molecular weight

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



