Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
λ-Cyhalothrin (Icon) is a type II synthetic pyrethroid insecticide featuring a high-efficiency, broad-spectrum formula with an α-cyano group. It is employed across various applications for controlling a wide array of pests. As a neurotoxin, λ-Cyhalothrin acts on sodium channels in neuron membranes within the central nervous system.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 50 mg | $30 | In Stock | In Stock | |
| 100 mg | $39 | In Stock | In Stock | |
| 500 mg | $85 | In Stock | In Stock | |
| 1 g | $123 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $44 | In Stock | In Stock |
| Description | λ-Cyhalothrin (Icon) is a type II synthetic pyrethroid insecticide featuring a high-efficiency, broad-spectrum formula with an α-cyano group. It is employed across various applications for controlling a wide array of pests. As a neurotoxin, λ-Cyhalothrin acts on sodium channels in neuron membranes within the central nervous system. |
| In vitro | λ-Cyhalothrin's toxicity mechanism involves delaying sodium channel inactivation, leading to prolonged depolarization of the nerve membrane. This compound causes membrane depolarization, influx of calcium ions, and neurotransmitter release in rat brain synaptosomes[1]. Additionally, λ-Cyhalothrin exhibits estrogenic qualities, acting as a xenoestrogen that promotes proliferation of human breast carcinoma cells in vitro[1]. It is extensively utilized for controlling pests (fleas, cockroaches, flies, and ants)[1] and is highly effective against Anopheles species, known for transmitting malaria[1]. |
| In vivo | The investigation assesses the impact of λ-Cyhalothrin (i.p.) on memory, movement activity, and coordination in mice subjected to bilateral clamping of the carotid arteries (BCCA). It was found that neither memory nor coordination was negatively affected by BCCA or λ-Cyhalothrin. However, a notable decline in exploratory locomotor activity was observed in the BCCA/LCH group, and spontaneous movement activity significantly decreased in the BCCA/λ-Cyhalothrin group. Furthermore, when exposed to λ-Cyhalothrin in the presence of BCCA, a decrease in motor activity was evident in mice during two subsequent 30-minute sessions[1]. |
| Synonyms | lambda-Cyhalothrin, Karate, Icon |
| Molecular Weight | 449.85 |
| Formula | C23H19ClF3NO3 |
| Cas No. | 91465-08-6 |
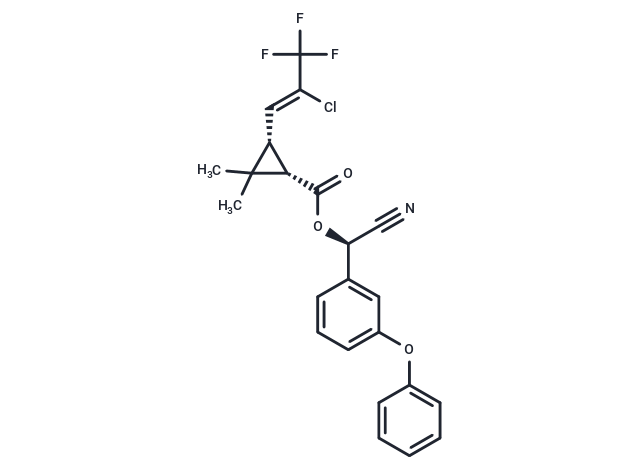
| Smiles | C(=C(/C(F)(F)F)\Cl)\[C@@H]1[C@H](C(O[C@@H](C#N)C2=CC(OC3=CC=CC=C3)=CC=C2)=O)C1(C)C |
| Relative Density. | 1.29. Temperature:20 °C. |
| Color | White |
| Appearance | Solid |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 250 mg/mL (555.74 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+90% Corn Oil: 2.5 mg/mL (5.56 mM), Sonication is recommeded. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.