 Your shopping cart is currently empty
Your shopping cart is currently empty

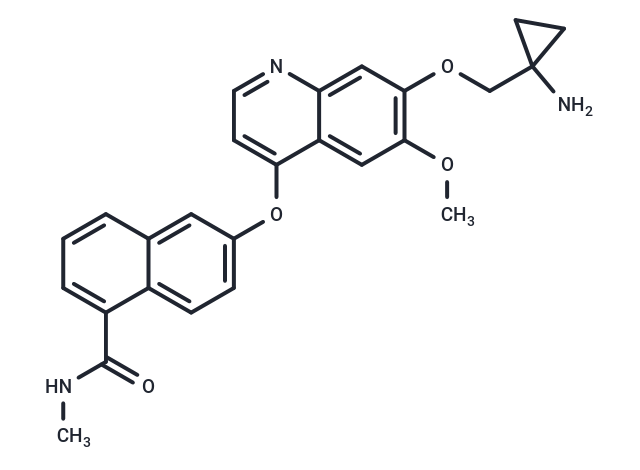
Lucitanib (E-3810) is a novel and potent inhibitor of VEGFR and FGFR, selectively targeting VEGFR1, VEGFR2, VEGFR3, FGFR1, and FGFR2 with IC50 values of 7 nM, 25 nM, 10 nM, 17.5 nM, and 82.5 nM, respectively.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $40 | In Stock | In Stock | |
| 2 mg | $57 | In Stock | In Stock | |
| 5 mg | $89 | In Stock | In Stock | |
| 10 mg | $147 | In Stock | In Stock | |
| 25 mg | $283 | In Stock | In Stock | |
| 50 mg | $513 | In Stock | In Stock | |
| 100 mg | $747 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $97 | In Stock | In Stock |
| Description | Lucitanib (E-3810) is a novel and potent inhibitor of VEGFR and FGFR, selectively targeting VEGFR1, VEGFR2, VEGFR3, FGFR1, and FGFR2 with IC50 values of 7 nM, 25 nM, 10 nM, 17.5 nM, and 82.5 nM, respectively. |
| Targets&IC50 | VEGFR1:7 nM, FGFR2:82.5 nM, VEGFR3:10 nM, FGFR1:17.5 nM, VEGFR2:25 nM |
| In vitro | Lucitanib potently inhibits FGFR2 activity (Ki<0.05 μM), follows by PDGFRα activity (Ki=0.11 μM) and it also potently inhibits VEGF and bFGF-stimulated HUVEC proliferation (IC50: 40 and 50 nM, respectively), which consistent with the inhibitory activity of VEGFR and FGFR auto-phosphorylation. Lucitanib (E-3810) also inhibits CSF-1R (IC50: 5 nM)[1].The Ki values obtained for DDR2, LYN, CARDIAK, CSBP (2), EPHA2, and YES range between 0.26 and 8 μM[2]. |
| In vivo | Lucitanib (20 mg/kg; 7 consecutive days; p.o.) treatment, completely inhibits (P<0.01) the bFGF induced angiogenic response compare with the response in vehicle-treated mice. E-3810 significantly delays growth during treatment, but tumors resume their growth when treatment is suspended; in a few cases, tumor regression is observed[1]. The activity of Lucitanib given at the doses of 15 mg/kg is tested on MDA-MB-231 breast cancer transplanted subcutaneously, at a late stage, when tumor masses reach 350 to 400 mg. This tumor xenograft is very sensitive to Lucitanib , with complete tumor stabilization lasting throughout the 30-day treatment. As in other tumor models, tumors re-grow after withdrawal of Lucitanib at a rate similar to control tumors[3]. Lucitanib displays a broad spectrum of activity, being active in all the xenografts tested (HT29 colon carcinoma, A2780 ovarian carcinoma, A498, SN12K1, and RXF393 renal carcinomas). It has dose-dependent inhibition of tumor growth. |
| Synonyms | E-3810 |
| Molecular Weight | 443.49 |
| Formula | C26H25N3O4 |
| Cas No. | 1058137-23-7 |
| Smiles | CNC(=O)c1cccc2cc(Oc3ccnc4cc(OCC5(N)CC5)c(OC)cc34)ccc12 |
| Relative Density. | 1.285 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 25 mg/mL (56.37 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (4.51 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.