 Your shopping cart is currently empty
Your shopping cart is currently empty

Oxybenzone is a UV filter commonly used in tanning and skin protection agents. It is a derivative of benzophenone and is used as an endocrine disrupting chemical that penetrates the placental and blood-brain barriers. It impairs autophagy, alters epigenetic status and disrupts vitamin X-like receptor signaling in apoptotic neuronal cells.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 g | $40 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock | In Stock |
| Description | Oxybenzone is a UV filter commonly used in tanning and skin protection agents. It is a derivative of benzophenone and is used as an endocrine disrupting chemical that penetrates the placental and blood-brain barriers. It impairs autophagy, alters epigenetic status and disrupts vitamin X-like receptor signaling in apoptotic neuronal cells. |
| In vitro | A dose-dependent inhibition of PGE2-production is found in the HEPM cell culture following oxybenzone exposure[1]. Benzophenones, including oxybenzone(BP-3) are documented mutagens that increase the rate of damage to DNA, especially when exposed to sunlight. It either can act directly as genotoxicants or become genotoxicants by bioactivation via cytochrome P450 enzymes. Oxybenzone can generate reactive oxygen species, which are potential mutagens, when applied topically to the skin followed by UV light exposure[2]. |
| In vivo | In mice studies, oxybenzone(BP-3) exposure significantly affects fecundity, as well as inducing unexplained mortality in lactating mothers. Studies in both mice and rats demonstrate that generational exposure to oxybenzone(BP-3) reduces body weight, increases liver ([50 %) and kidney weights, induces a 30 % increase in prostate weight, a reduction in immunocompetence, and significantly increases uterine weight in juveniles. In mammals, oxybenzone(BP-3) is renowned for having estrogenic and anti-androgenic activities, causing activation of estrogen receptor proteins and inhibition of androgen receptors[2]. |
| Cell Research | Cells are grown at 37℃ with 100% humidity and 10% CO2. Prior to analyses, cells are subcultured onto 24-well plates and allowed to become 70-90% confluent. On day 1 of the experiment, cells are washed three times with serum-free media containing 0.5% bovine serum albumin (BSA), followed by replacement with the same media that also contained 1 ng/ml of IL-1β with or without oxybenzone. After an exposure time of 24 h, the media are removed, the pH adjusted to 3.5 with HCl, and finally assayed for PGE2-production. (Only for Reference) |
| Synonyms | KAHSCREEN BZ-3, Eusolex 4360, Escalol 567, Benzophenone 3 |
| Molecular Weight | 228.24 |
| Formula | C14H12O3 |
| Cas No. | 131-57-7 |
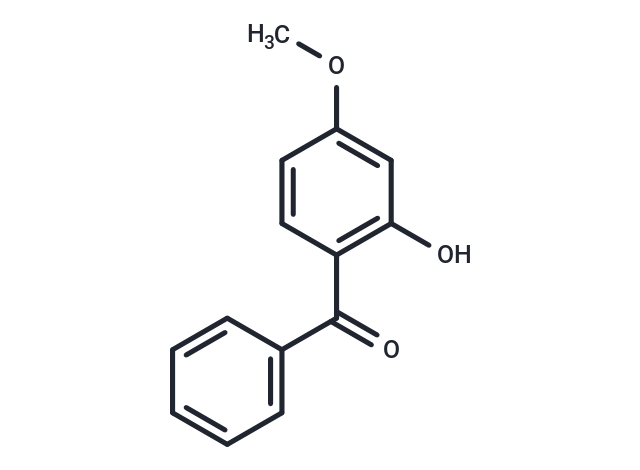
| Smiles | COC1=CC(O)=C(C=C1)C(=O)C1=CC=CC=C1 |
| Relative Density. | 1.3 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (219.07 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (8.76 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.