 Your shopping cart is currently empty
Your shopping cart is currently empty

NS309 is a nonselective activator of small- and intermediate-conductance Ca2+-activated K+ (SK/KCa2 and IK/KCa3.1) channels (EC50s range from 0.12-1.2 μM for SK channels and 10-90 nM for IK channels). It can also activate voltage-gated Kv11.1 channels (hERG; IC50 = 1 μM), but does not, however, affect large conductance (BK/KCa1.1) channels. NS309 can modulate neuronal firing patterns by enhancing SK-mediated hyperpolarizing effects. It is 730-fold (SK channels) and 7,400-fold (IK channels) more potent than 1-EBIO and often used in conjunction with CyPPA to distinguish between SK1 and SK3 subtypes.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 mg | $52 | In Stock | In Stock | |
| 10 mg | $63 | In Stock | In Stock | |
| 25 mg | $122 | In Stock | In Stock | |
| 50 mg | $228 | In Stock | In Stock | |
| 100 mg | $339 | In Stock | In Stock | |
| 200 mg | $495 | In Stock | In Stock | |
| 500 mg | $791 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $57 | In Stock | In Stock |
| Description | NS309 is a nonselective activator of small- and intermediate-conductance Ca2+-activated K+ (SK/KCa2 and IK/KCa3.1) channels (EC50s range from 0.12-1.2 μM for SK channels and 10-90 nM for IK channels). It can also activate voltage-gated Kv11.1 channels (hERG; IC50 = 1 μM), but does not, however, affect large conductance (BK/KCa1.1) channels. NS309 can modulate neuronal firing patterns by enhancing SK-mediated hyperpolarizing effects. It is 730-fold (SK channels) and 7,400-fold (IK channels) more potent than 1-EBIO and often used in conjunction with CyPPA to distinguish between SK1 and SK3 subtypes. |
| Targets&IC50 | ERG (human):1 µM |
| Molecular Weight | 231.04 |
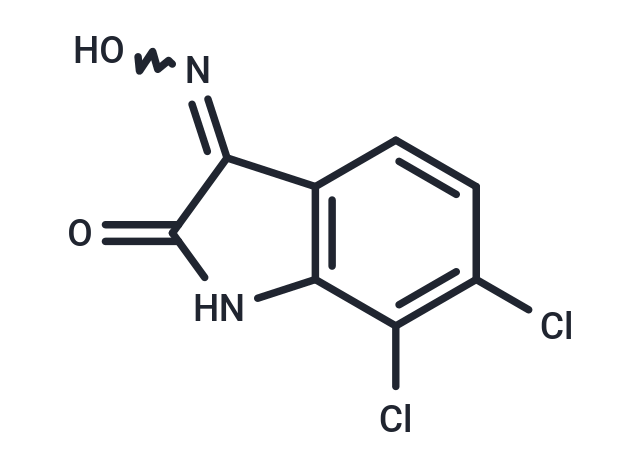
| Formula | C8H4Cl2N2O2 |
| Cas No. | 18711-16-5 |
| Smiles | ON=C1C(=O)Nc2c1ccc(Cl)c2Cl |
| Relative Density. | no data available |
| Color | Yellow |
| Appearance | Solid |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 45 mg/mL (194.77 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.