Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Monensin sodium is an antiprotozoal agent produced by Streptomyces cinnamonensis.Monensin sodium salt (Monensin A sodium salt) causes a marked enlargement of the MVBs and regulates exosome secretion
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 50 mg | $30 | In Stock | In Stock | |
| 100 mg | $42 | In Stock | In Stock | |
| 200 mg | $58 | In Stock | In Stock |
| Description | Monensin sodium is an antiprotozoal agent produced by Streptomyces cinnamonensis.Monensin sodium salt (Monensin A sodium salt) causes a marked enlargement of the MVBs and regulates exosome secretion |
| In vitro | Monensin sodium salt (Coban), isolated from Streptomyces cinnamonensis, is a well-known representative of naturally polyether ionophore antibiotics. Monensin is an ionophore related to the crown ethers with a preference to form complexes with monovalent cations such as: Li+, Na+, K+, Rb+, Ag+, and TI+. [1] Monensin A is able to transport these cations across lipid membranes of cells, playing an important role as an Na+/H+ antiporter. It blocks intracellular protein transport, and exhibits antibiotic, antimalarial, and other biological activities. [2] The antibacterial properties of monensin and its derivatives are a result of their ability to transport metal cations through cellular and subcellular membranes. [3] |
| In vivo | LD50: Mice 335 mg/kg (i.g.) [4]; Rats 36.5 mg/kg (i.g.); Chickens 185 mg/kg (i.g.). [5] |
| Synonyms | Sodium Monensin, Monensin A sodium salt |
| Molecular Weight | 692.85 |
| Formula | C36H61NaO11 |
| Cas No. | 22373-78-0 |
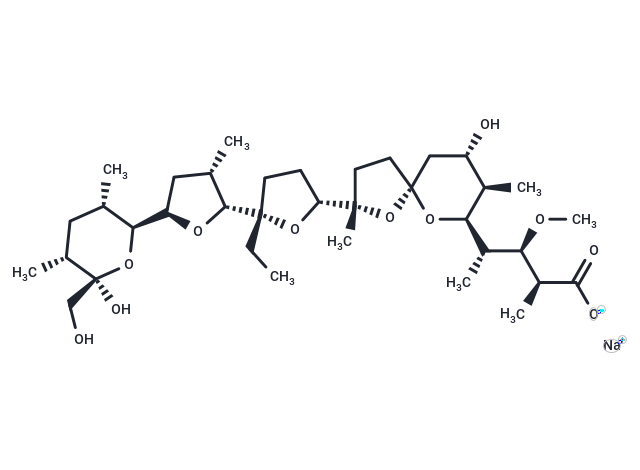
| Smiles | [Na+].CC[C@]1(CC[C@@H](O1)[C@]1(C)CC[C@]2(C[C@H](O)[C@@H](C)[C@H](O2)[C@@H](C)[C@@H](OC)[C@H](C)C([O-])=O)O1)[C@@H]1O[C@H](C[C@@H]1C)[C@H]1O[C@@](O)(CO)[C@H](C)C[C@@H]1C |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||
| Solubility Information | Ethanol: 12.5 mg/mL (18.04 mM), Sonication is recommended. DMSO: < 1 mg/mL (insoluble or slightly soluble) | ||||||||||||||||||||
| In Vivo Formulation | 10% EtOH+90% Corn Oil: 1 mg/mL (1.44 mM), Sonication is recommeded. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
Ethanol
| |||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.