Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
(+)-BORNEOL (d-Borneol) is a natural bicyclic monoterpene used for analgesia and anesthesia in traditional Chinese medicine; enhances GABA receptor activity with an EC50 of 248 μM.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 50 mg | $50 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock | In Stock |
| Description | (+)-BORNEOL (d-Borneol) is a natural bicyclic monoterpene used for analgesia and anesthesia in traditional Chinese medicine; enhances GABA receptor activity with an EC50 of 248 μM. |
| Targets&IC50 | GABA:248 μM(EC50) |
| In vitro | Aβ-induced cell cytotoxicity was inhibited by 100 μM of (-) and (+) borneol treatment. Treatment of borneol significantly decreased ROS generation (P < 0.01). The expression of HO-1 and nuclear translocation of Nrf2 were increased by Aβ treatment. This nuclear translocation of Nrf2 was further increased by administration of borneol. Compared with the Aβ treated group, the (+) borneol treated group significantly increased Bcl-2 expression with decreased expression of Bax[1]. |
| In vivo | (+)-borneol (1.0 mg/kg) significantly ameliorated infarct size and neurological scoresvia reducing the expression of inducible nitric oxide synthase (iNOS) and tumor necrosis factor-alpha (TNF-α) in a dose dependent manner. Notably, (+)-borneol showed long-term effects on the improvement of sensorimotor functions in the photothrombotic model of stroke, which decreased the number of foot faults in the grid-walking task and forelimb asymmetry scores in the cylinder task, at least in part through reducing loss of dendritic spines in the length, brunch number and density. Suggest that (+)-borneol could serve as a therapeutic target for ischemic stroke[2]. |
| Cell Research | Oxidative stress was induced by administering 50 μM Aβ into SH-SY5Y cells. Neuroprotective effect of commercially available borneol was examined by determining cell viability with the MTT assay. Intracellular reactive oxygen species (ROS) generation was measured using a fluorometer with further examination of heme oxygenase-1 (HO-1) and nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) expression. Apoptosis was examined by measuring the ratio of B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax)[1]. |
| Animal Research | To mimic a typical human stroke, which does not undergo reperfusion, used a permanent MCAO in this study. For investigating the role of (+)-borneol in permanent cerebral ischemia, 45 male Sprague-Dawley rats were randomly divided into the sham group, the vehicle-treated group and the (+)-borneol-treated groups (1.0 mg/kg), with 15 rats in each group. The terminal half-life (t1/2) of borneol was 2 h following cerebral ischemia-reperfusion. We subjected the rats to pMCAO and administered drugs by tail intravenous injection 2 hours and 5 hours after pMCAO, respectively. The sham group and the vehicle-treated group were injected with vehicle administration. Neurologic scores were assessed at 48 hours after reperfusion[2]. |
| Synonyms | d-Borneol |
| Molecular Weight | 154.25 |
| Formula | C10H18O |
| Cas No. | 464-43-7 |
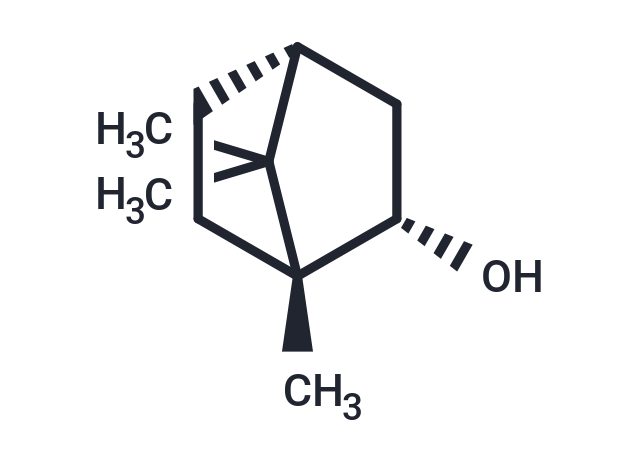
| Smiles | CC1(C)[C@@H]2CC[C@@]1(C)[C@@H](O)C2 |
| Relative Density. | 0.992g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 247.5 mg/mL (1604.54 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 4 mg/mL (25.93 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.