 Your shopping cart is currently empty
Your shopping cart is currently empty

Olmesartan (RNH 6270) is an Angiotensin II Type I receptor antagonist. Olmesartan is the active form of the antihypertensive drug olmesartan medoxomil,and has antihypertensive activity.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 25 mg | $39 | In Stock | In Stock | |
| 50 mg | $64 | In Stock | In Stock | |
| 100 mg | $98 | - | In Stock | |
| 500 mg | $262 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $43 | In Stock | In Stock |
| Description | Olmesartan (RNH 6270) is an Angiotensin II Type I receptor antagonist. Olmesartan is the active form of the antihypertensive drug olmesartan medoxomil,and has antihypertensive activity. |
| In vivo | The target blood pressure (<130/80 mm Hg) was achieved in nearly 80% of the patients taking olmesartan and 71% taking placebo;?blood pressure measured in the clinic was lower by 3.1/1.9 mm Hg in the olmesartan group than in the placebo group.?Microalbuminuria developed in 8.2% of the patients in the olmesartan group (178 of 2160 patients who could be evaluated) and 9.8% in the placebo group (210 of 2139);?the time to the onset of microalbuminuria was increased by 23% with olmesartan (hazard ratio for onset of microalbuminuria, 0.77;?95% confidence interval, 0.63 to 0.94;?P=0.01).?The serum creatinine level doubled in 1% of the patients in each group.?Slightly fewer patients in the olmesartan group than in the placebo group had nonfatal cardiovascular events--81 of 2232 patients (3.6%) as compared with 91 of 2215 patients (4.1%) (P=0.37)--but a greater number had fatal cardiovascular events--15 patients (0.7%) as compared with 3 patients (0.1%) (P=0.01),?a difference that was attributable in part to a higher rate of death from cardiovascular causes in the olmesartan group than in the placebo group among patients with preexisting coronary heart disease (11 of 564 patients [2.0%] vs. 1 of 540 [0.2%], P=0.02)[1]. |
| Animal Research | In a randomized, double-blind, multicenter, controlled trial, Assigned 4447 patients with type 2 diabetes to receive olmesartan (at a dose of 40 mg once daily) or placebo for a median of 3.2 years.?Additional antihypertensive drugs (except angiotensin-converting-enzyme inhibitors or ARBs) were used as needed to lower blood pressure to less than 130/80 mm Hg. The primary outcome was the time to the first onset of microalbuminuria.?The times to the onset of renal and cardiovascular events were analyzed as secondary end points[1]. |
| Synonyms | RNH 6270, CS 088 |
| Molecular Weight | 446.50 |
| Formula | C24H26N6O3 |
| Cas No. | 144689-24-7 |
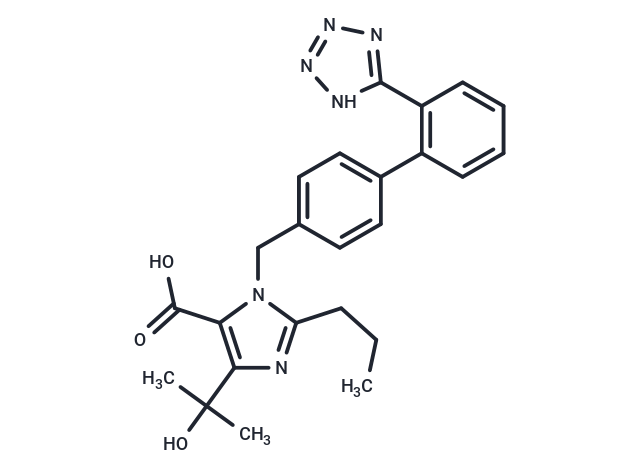
| Smiles | C(N1C(C(O)=O)=C(C(C)(C)O)N=C1CCC)C2=CC=C(C=C2)C3=C(C=CC=C3)C=4NN=NN4 |
| Relative Density. | 1.33 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||
| Solubility Information | DMSO: 6.25 mg/mL (14.00 mM), Sonication is recommended. | ||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 1.00 mg/mL (2.24 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.