 Your shopping cart is currently empty
Your shopping cart is currently empty

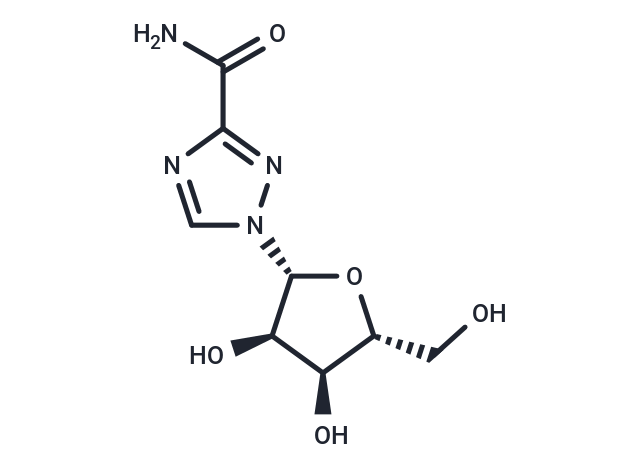
Ribavirin (Tribavirin) is a synthetic nucleoside analog of ribofuranose with activity against hepatitis C virus and other RNA viruses.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 25 mg | $30 | In Stock | In Stock | |
| 50 mg | $39 | In Stock | In Stock | |
| 100 mg | $48 | In Stock | In Stock | |
| 500 mg | $163 | In Stock | In Stock | |
| 1 g | $238 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock | In Stock |
| Description | Ribavirin (Tribavirin) is a synthetic nucleoside analog of ribofuranose with activity against hepatitis C virus and other RNA viruses. |
| Targets&IC50 | Macrophages (anti-inflammatory):19 μM, K562 cells:78.30 µM, A549 cells:3 μM (EC50), Macrophages (anti-inflammatory):7 μM (EC50), SUP-B15 cells:65.18 µmol/ L |
| In vivo | METHODS: To investigate the antiviral effect against CCHF virus, Ribavirin (100 mg/kg) was administered intraperitoneally to CCHF virus-infected IFNAR-/- mice once daily until death or day 8. RESULTS: CCHF virus-infected IFNAR-/- mice died 2-6 days post-infection, with elevated blood and organ transaminase levels and high viral titers.Ribavirin did not increase the survival rate of the IFNAR-/- mice but prolonged the time to death and decreased the transaminase levels and viral titers. [3] |
| Cell Research | The effect of Ribavirin on microglial cell viability is evaluated by the sulforhodamine B (SRB) chemosensitivity assay. Briefly, LPS-stimulated microglial cells are incubated for 48 h in the presence or absence of Ribavirin. Afterward, the cells are fixed in 10% (w/v) trichloroacetic acid for 1 h at 4°C, rinsed in tap water and stained with 0.4% (w/v) SRB in 1% acetic acid (100 μL/well) for 30 min at room temperature (RT). The cells are then rinsed three times in 1% acetic acid to remove the unbound stain. The protein bound stain is extracted with 200 μL 10 mM Tris base (pH 10.5) per well. The optical density is read at 540 nm, with correction at 670 nm. The results are presented as percentage of the control (non-stimulated/untreated microglial cells), that is arbitrarily set to 100%. |
| Synonyms | Tribavirin, RTCA, NSC-163039, ICN-1229 |
| Molecular Weight | 244.20 |
| Formula | C8H12N4O5 |
| Cas No. | 36791-04-5 |
| Smiles | NC(=O)C1=NN(C=N1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |
| Relative Density. | 2.08 g/cm3 |
| Storage | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 24.4 mg/mL (99.92 mM), Sonication is recommended. DMSO: 257.5 mg/mL (1054.46 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5 mg/mL (20.48 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
H2O/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.