 Your shopping cart is currently empty
Your shopping cart is currently empty

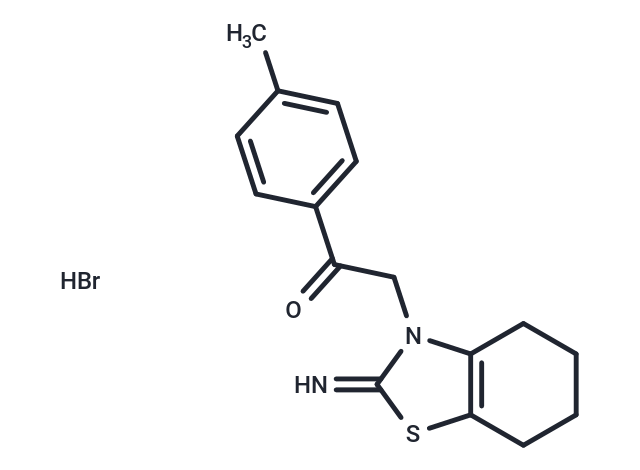
Pifithrin-α hydrobromide (Pifithrin-α hydrobromide) is a p53 inhibitor, inhibiting p53-dependent transactivation of p53-responsive genes.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 mg | $33 | In Stock | In Stock | |
| 5 mg | $52 | In Stock | In Stock | |
| 10 mg | $81 | In Stock | In Stock | |
| 25 mg | $180 | In Stock | In Stock | |
| 50 mg | $293 | In Stock | In Stock | |
| 100 mg | $438 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $54 | In Stock | In Stock |
| Description | Pifithrin-α hydrobromide (Pifithrin-α hydrobromide) is a p53 inhibitor, inhibiting p53-dependent transactivation of p53-responsive genes. |
| In vitro | At a concentration of 10 μM, Pifithrin-α inhibits apoptosis in C8 cells induced by Dox, Etoposide, Taxol, and Cytosine arabinoside. It also reduces activation of the heat shock transcription factor and enhances cell sensitivity to heat, lowers glucocorticoid receptor activation in HeLa cells, and protects murine thymocytes from apoptosis induced by Dexamethasone. Concentrations between 100–200 nM of Pifithrin-α completely block the increase in p53 DNA binding levels and the p53-responsive gene Bax in hippocampal cells induced by Camptothecin, while 200 nM safeguards cultured hippocampal neurons from death caused by DNA damage agents. Furthermore, 200 μM Pifithrin-α stabilizes mitochondrial function, inhibits caspase activation, and shields hippocampal neurons from death induced by glutamate and β-amyloid peptide. Pifithrin-α prevents the p53-dependent growth inhibition in human diploid fibroblasts following DNA damage, though it does not affect fibroblasts lacking p53. It can regulate the nuclear import and export of p53, or both, and decrease nuclear p53 stability. Pifithrin-α inhibits signals from heat shock and glucocorticoid receptors without affecting NF-κB signaling. |
| In vivo | Intraperitoneal injection of 3.6 μg/kg Pifithrin-α in mice significantly inhibited Dex-induced thymic atrophy. Compared to the control group, Pifithrin-α (2 mg/kg) notably decreased the extent of motor dysfunction in rats with transient cerebral artery occlusion. Administration of Pifithrin-α (2 mg/kg i.p.) 30 minutes before treatment of cerebral artery occlusion in mice reduced ischemic brain damage and shielded hippocampal neurons from excitotoxicity damage. In C57BL and Balb/c mice, intraperitoneal injection of 2.2 mg/kg Pifithrin-α completely protected the mice against the lethal effects of 60% mortality gamma-ray irradiation. Pifithrin-α was observed to substantially lower cellular apoptosis in rats, evidenced by Tunel and caspase 3 staining. When administered within one hour after a stroke, animals treated with Pifithrin-α exhibited fewer motor dysfunctions and smaller infarcts. After 7 days of treatment with Pifithrin-α, rats showed significantly reduced scores of motor dysfunction compared to the placebo control group. |
| Kinase Assay | The ligand binding competition assays are performed. Cytosolic cell extracts from Hepa-1 cells are generated by the resuspension of the cell pellets in HEDG buffer [25 mM Hepes, 1 mM EDTA, 1 mM dithiothreitol, and 10% (v/v) glycerol, pH 7.5] containing 0.4 mM leupeptin, 4 mg/mL aprotinin, and 0.3 mM phenylmethylsulfonyl fluoride, homogenization, and centrifugation at 100,000 g for 45 min. Aliquots of the supernatant (120 μg) are incubated at room temperature for 2 h with the indicated concentrations of Pifithrin-α in the presence of 3 nM [3H]TCDD in HEDG buffer. After incubation on ice with hydroxyapatite for 30 min, HEDG buffer with 0.5% Tween 80 is added. The samples are centrifuged, washed twice, resuspended in 0.2 mL of scintillation fluid, and subjected to scintillation counting. Nonspecific binding is determined using a 150-fold molar excess of TCDF and subtracted from the total binding to obtain the specific binding. The specific binding is reported relative to [3H]TCDD alone[2]. |
| Cell Research | At the end of cell treatments, the number of attached cells is estimated by staining with 0.25% crystal violet in 50% methanol, followed by elution of the dye with 1% SDS. Optical density (530 nm) reflecting the number of stained cells is determined with a Bio-Tek EL311 microplate reader. Cell viability in suspension of short term culture of primary thymocytes is determined by their staining with 0.1% of methyl blue and microscopic counting of blue (dead) cells.(Only for Reference) |
| Synonyms | Pifithrin-α (PFTα) HBr, Pifithrin-α, Pifithrin hydrobromide, PFTα hydrobromide, PFTα |
| Molecular Weight | 367.3 |
| Formula | C16H18N2OS·HBr |
| Cas No. | 63208-82-2 |
| Smiles | Br.Cc1ccc(cc1)C(=O)Cn1c2CCCCc2sc1=N |
| Relative Density. | 1.28g/cm3 |
| Storage | keep away from moisture,store at low temperature | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (136.13 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (5.45 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.