Powder: -20°C for 3 years | In solvent: -80°C for 1 year


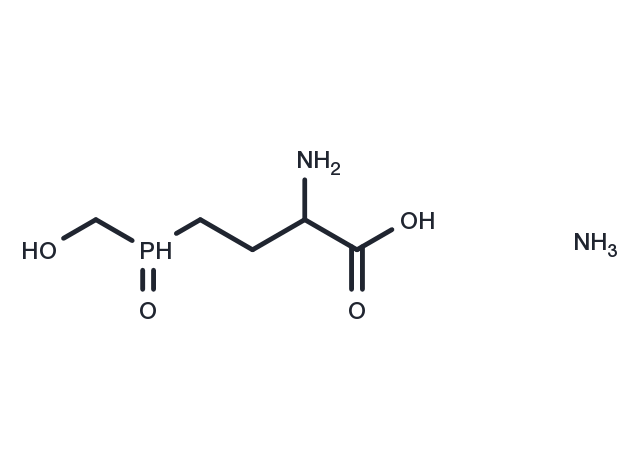
Glufosinate ammonium is a phosphinic acid analogue of glutamic acid and is an herbicide. Glufosinate ammonium is converted by plant cells into PT (L-phosphinothricin). Glufosinate ammonium has neurotoxic activity.
| Pack Size | Availability | Price/USD | Quantity |
|---|---|---|---|
| 100 mg | In stock | $ 66.00 |


| Description | Glufosinate ammonium is a phosphinic acid analogue of glutamic acid and is an herbicide. Glufosinate ammonium is converted by plant cells into PT (L-phosphinothricin). Glufosinate ammonium has neurotoxic activity. |
| In vitro | Glufosinate ammonium impairs ependymal cell capability to synthetize cilia. Glufosinate ammonium (1-100 μM; 12DIV) obviously reduces Celsr2 gene expression at 100 μM. Glufosinate ammonium (1-100 μM; 12DIV) disrupts cell-cell adhesion in differentiated V-SVZ neural stem cells (NSCs) [2]. |
| In vivo | Glufosinate ammonium (10-250 mg/kg; gavage; Daily; on days 6-15 of gestation) shows maternal toxicity in the groups given 50 or 250 mg/kg in Wistar rats. In male and female rats, the Elimination of Glufosinate ammonium (oral intubation) in the blood takes place with a half-life of fewer than 4 hours [3]. |
| Molecular Weight | 198.16 |
| Formula | C5H15N2O4P |
| CAS No. | 77182-82-2 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
H2O: 250 mg/mL (1261.61 mM), Sonication is recommended.
You can also refer to dose conversion for different animals. More
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
Glufosinate ammonium 77182-82-2 Others cell adhesion analogue Glufosinate stem neurotoxic acid inhibit differentiated phosphinic neural Inhibitor Celsr2 plant inhibitor
