 Your shopping cart is currently empty
Your shopping cart is currently empty

Emricasan (IDN-6556) is a pan-caspase inhibitor with irreversible properties. Emricasan has anti-inflammatory and anti-apoptotic activity and can be used in the treatment of infections and liver failure, etc. Emricasan also inhibits Zika virus infections.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 mg | $50 | In Stock | In Stock | |
| 5 mg | $83 | In Stock | In Stock | |
| 10 mg | $140 | In Stock | In Stock | |
| 25 mg | $253 | In Stock | In Stock | |
| 50 mg | $419 | In Stock | In Stock | |
| 100 mg | $617 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $103 | In Stock | In Stock |
| Description | Emricasan (IDN-6556) is a pan-caspase inhibitor with irreversible properties. Emricasan has anti-inflammatory and anti-apoptotic activity and can be used in the treatment of infections and liver failure, etc. Emricasan also inhibits Zika virus infections. |
| Targets&IC50 | Caspase-8:0.4 nM, THP-1 cells:0.27 μM, Caspase-3:0.31 nM, JFas cells:0.025 μM |
| In vitro | METHODS: Primary rat cirrhotic hepatocytes were treated with Emricasan (50 µM) for 24 h, and mRNA expression levels were measured. RESULTS: Emricasan directly improved the expression of hepatocyte-specific markers. [1] METHODS: Human cervical cancer cells HeLa were pretreated with Emricasan (10 µM) for 1 h, then treated with vincristine (20 nM) for 44 h. Cell death was detected by Flow Cytometry. RESULTS: Emricasan eliminated 95% of vincristine-mediated cell death. [2] |
| In vivo | METHODS: To study the effects on chronic liver disease, Emricasan (10 mg/kg, 0.9% dimethylcarboxycellulose) was administered orally once daily for seven days to rats with advanced cirrhosis due to chronic CCl4 administration. RESULTS: Emricasan ameliorated hepatic sinusoidal microvascular dysfunction in cirrhotic patients, resulting in significant improvement in fibrosis, portal hypertension and liver function. [1] METHODS: To investigate the effects on cirrhosis, Emricasan (10 mg/kg) was administered intraperitoneally once daily for 10-20 days to C57BL/6 mice with secondary biliary cirrhosis induced by bile duct ligation (BDL). RESULTS: Emricasan treatment improved survival and portal hypertension (PHT) in a mouse model of long-term BDL. [3] |
| Cell Research | Astrocytes are mock-infected, treated with DMSO or treated with 2 μM niclosamide, 92 μM PHA-690509, 9 μM emricasan, or a combination of 92 μM PHA-690509 and 9 μM emricasan for 1 h before infection with PRVABC59 (MOI = 0.5). Cells are fixed 24 h after infection and stained for ZIKVE and nuclei.(Only for Reference) |
| Synonyms | PF 03491390, IDN-6556 |
| Molecular Weight | 569.5 |
| Formula | C26H27F4N3O7 |
| Cas No. | 254750-02-2 |
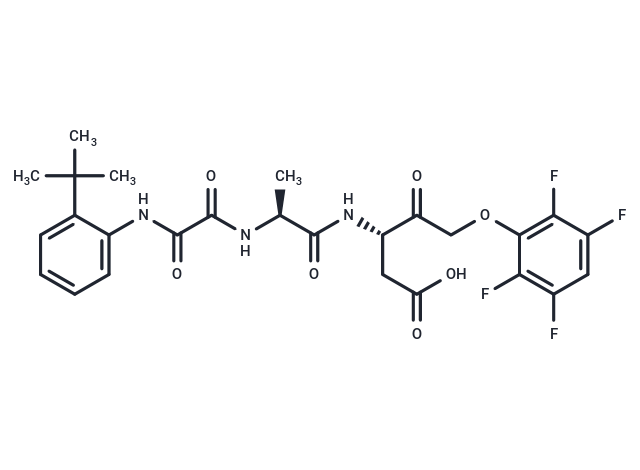
| Smiles | C[C@H](NC(=O)C(=O)Nc1ccccc1C(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1c(F)c(F)cc(F)c1F |
| Relative Density. | 1.386 g/cm3 |
| Storage | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: < 1 mg/mL (insoluble or slightly soluble) DMSO: 257.5 mg/mL (452.15 mM), Sonication is recommended. Ethanol: 93 mg/mL (163.3 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5 mg/mL (8.78 mM), Solution. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.