Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
DMBA (7,12-Dimethylbenz[a]anthracene) is a carcinogenic polycyclic aromatic hydrocarbon (PAH) that can be used to induce animal models of leukemia, liver cancer, breast cancer, skin cancer, and lung cancer. It is also capable of inducing programmed cell death (apoptosis) in A20.1 murine B-cell lymphoma.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $30 | In Stock | |
| 50 mg | $40 | In Stock | |
| 100 mg | $64 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $39 | In Stock |
| Description | DMBA (7,12-Dimethylbenz[a]anthracene) is a carcinogenic polycyclic aromatic hydrocarbon (PAH) that can be used to induce animal models of leukemia, liver cancer, breast cancer, skin cancer, and lung cancer. It is also capable of inducing programmed cell death (apoptosis) in A20.1 murine B-cell lymphoma. |
| In vivo | DMBA (0-150 mg/kg, oral administration) induces a decrease in spleen weight and a reduction in the total number of lymphocytes recovered from the spleen in C57BL/6 mice. This model can be used to establish induced cancer models such as skin carcinogenesis, breast cancer, and lung cancer[5]. |
| Synonyms | 7,12-DMBA, 7,12-Dimethylbenzanthracene, 7,12-Dimethylbenz[a]anthracene |
| Molecular Weight | 256.34 |
| Formula | C20H16 |
| Cas No. | 57-97-6 |
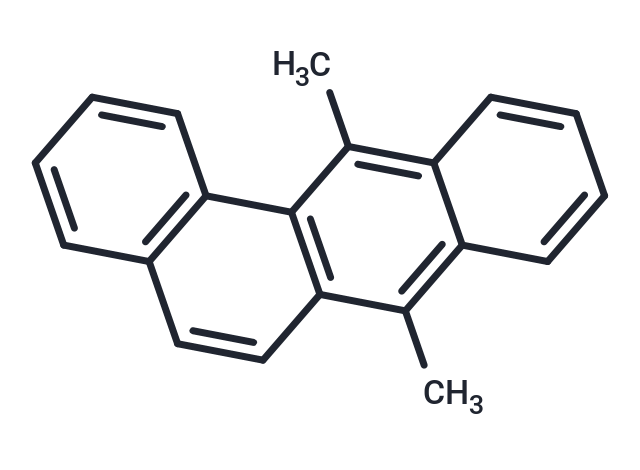
| Smiles | Cc1c2ccccc2c(C)c2c1ccc1ccccc21 |
| Color | Yellow |
| Appearance | Solid |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 15 mg/mL (58.5 mM), Sonification is recommended. DMF: 20 mg/mL (78.02 mM), Sonication is recommended. Acetone: 15 mg/mL (58.5 mM), Sonification is recommended. Ethanol: 2 mg/mL (7.80 mM), Sonication and heating are recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
Ethanol/DMSO/Acetone/DMF
DMSO/Acetone/DMF
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.