 Your shopping cart is currently empty
Your shopping cart is currently empty

Lomitapide (AEGR-733) is a small molecule inhibitor of microsomal triglyceride transfer protein (MTP), an enzyme located in the lumen of the endoplasmic reticulum responsible for absorbing dietary lipids and transferring triglycerides onto apolipoprotein B (apo-B) in the assembly of very-low-density lipoprotein.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 mg | $59 | In Stock | In Stock | |
| 5 mg | $96 | In Stock | In Stock | |
| 10 mg | $148 | In Stock | In Stock | |
| 25 mg | $243 | In Stock | In Stock | |
| 50 mg | $349 | In Stock | In Stock | |
| 100 mg | $525 | In Stock | In Stock | |
| 200 mg | $754 | In Stock | In Stock | |
| 500 mg | $1,160 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $164 | In Stock | In Stock |
| Description | Lomitapide (AEGR-733) is a small molecule inhibitor of microsomal triglyceride transfer protein (MTP), an enzyme located in the lumen of the endoplasmic reticulum responsible for absorbing dietary lipids and transferring triglycerides onto apolipoprotein B (apo-B) in the assembly of very-low-density lipoprotein. |
| Targets&IC50 | MTP:8 nM |
| In vitro | Lomitapide is an oral microsomal triglyceride transfer protein (MTP) inhibitor indicated for the treatment of patients with HoFH, a rare form of hypercholesterolemia that can lead to premature atherosclerotic disease. Lomitapide undergoes hepatic metabolism through cytochrome P-450 (CYP) isoenzyme 3A4 and interacts with CYP3A4 substrates including atorvastatin and simvastatin[2]. |
| In vivo | Lomitapide, either as monotherapy or in combination with other lipid-lowering agents, notably reduces low-density lipoprotein cholesterol (LDL-C) levels by over 50%. However, its use is accompanied by significant gastrointestinal side effects and increased hepatic fat. The 50-mg capsule of lomitapide has a bioavailability of 7.1%, and its mean half-life is approximately 39.7 hours[2]. Administration of a single dose of lomitapide can decrease serum triglycerides by 35% and 47% at dosages of 0.3 and 1 mg/kg, respectively. Moreover, multiple-dose regimens have shown a dose-dependent reduction in triglycerides (71%-87%), nonesterified fatty acids (33%-40%), and LDL-C (26-29%)[3]. |
| Synonyms | BMS-201038, AEGR-733 |
| Molecular Weight | 693.72 |
| Formula | C39H37F6N3O2 |
| Cas No. | 182431-12-5 |
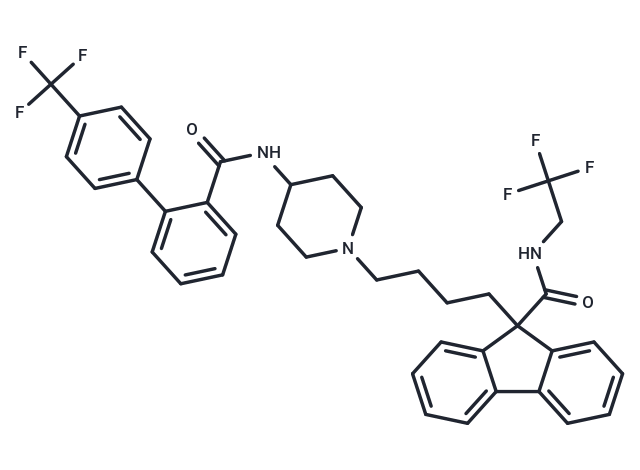
| Smiles | FC(F)(F)CNC(=O)C1(CCCCN2CCC(CC2)NC(=O)c2ccccc2-c2ccc(cc2)C(F)(F)F)c2ccccc2-c2ccccc12 |
| Relative Density. | 1.34 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 242 mg/mL (348.84 mM), Sonication is recommended. H2O: < 1 mg/mL (insoluble or slightly soluble) Ethanol: 93 mg/mL (134.06 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5 mg/mL (7.21 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.