 Your shopping cart is currently empty
Your shopping cart is currently empty

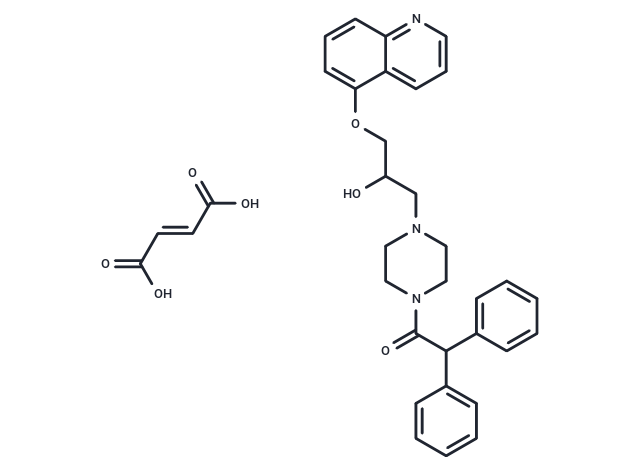
Dofequidar fumarate (MS-209)(MS-209 fumarate), a quinoline-based compound administered orally, is known for counteracting multidrug resistance (MDR) through the inhibition of ABCB1/P-glycoprotein (P-gp) and ABCC1/MDR-associated protein 1.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $33 | In Stock | In Stock | |
| 2 mg | $46 | In Stock | In Stock | |
| 5 mg | $77 | In Stock | In Stock | |
| 10 mg | $127 | In Stock | In Stock | |
| 25 mg | $258 | In Stock | In Stock | |
| 50 mg | $497 | In Stock | In Stock | |
| 100 mg | $723 | In Stock | In Stock | |
| 500 mg | $1,520 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $97 | In Stock | In Stock |
| Description | Dofequidar fumarate (MS-209)(MS-209 fumarate), a quinoline-based compound administered orally, is known for counteracting multidrug resistance (MDR) through the inhibition of ABCB1/P-glycoprotein (P-gp) and ABCC1/MDR-associated protein 1. |
| In vitro | Dofequidar fumarate effectively overcomes docetaxel resistance in MDR cancer cells, and this concentration reaches> 7 h in plasma without severe toxicity.Dofequidar fumarate restored the chemical sensitivity of SBC-3/ADM cells to VP-16, ADM and VCR in a dose-dependent manner in vitro. Dofequidar inhibits the outflow of chemotherapy drugs and increases the sensitivity of anti-cancer drugs to CSC-like side population (SP) cells isolated from various cancer cell lines. Dofequidar treatment greatly reduces the number of cells in the SP component. In 4-1St cells with strong resistance to ADM and VCR, Dofequidar fumarate at a concentration of 3 microM increased the cytotoxicity of ADM and VCR by 88-fold and 350-fold, respectively. |
| In vivo | Docetaxel alone at the maximum tolerated dose (MTD) has significant antitumor activity against intrinsically resistant HCT-15 tumor xenografts, while Dofequidar fumarate also enhances the antitumor effect of docetaxel active. For MCF-7/ADM tumor xenografts expressing large amounts of P-gp, docetaxel alone did not show antitumor activity at MTD, while the combination of MTD and Dofequidar fumarate for docetaxel greatly reduced MCF -7/ADM tumor growth. Intravenous injection of SBC-3 or SBC-3/ADM cells will produce metastatic colonies in the liver, kidneys, and lymph nodes in severe combined immunodeficiency (SCID) mice depleted by natural killer (NK) cells, although SBC-3/ ADM cells produce faster transfers than SBC-3 cells. The treatment of VP-16 and ADM reduced the formation of metastasis of SBC-3 cells, while the same treatment did not affect the metastasis of SBC-3/ADM cells. Although the use of MS-209 alone has no effect on the transfer of SBC-3 or SBC-3/ADM cells, the combined use of MS-209 and VP-16 or ADM can significantly inhibit the proliferation of SBC-3/ADM cells Metastasis to form an organ. |
| Synonyms | MS-209 |
| Molecular Weight | 597.66 |
| Formula | C34H35N3O7 |
| Cas No. | 153653-30-6 |
| Smiles | OC(=O)\C=C\C(O)=O.OC(COc1cccc2ncccc12)CN1CCN(CC1)C(=O)C(c1ccccc1)c1ccccc1 |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 1 mg/mL (1.67 mM), Sonication is recommended. DMSO: 100 mg/mL (167.32 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 4 mg/mL (6.69 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
H2O/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.