Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Pyranine (Solvent Green 7) is reported as a new class of fluorescent chemosensor for the Cu+ ion.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 500 mg | $46 | In Stock | In Stock | |
| 1 g | $79 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $51 | In Stock | In Stock |
| Description | Pyranine (Solvent Green 7) is reported as a new class of fluorescent chemosensor for the Cu+ ion. |
| In vitro | I. Cu2+ ion detection 1. Material preparation: 1) Pyranine solution: Pyranine is usually dissolved in an appropriate solvent, such as water, PBS or other buffer, to a concentration of 1-10 μM. 2) Cu+ ion solution: Prepare Cu+ ion solutions of different concentrations. 3) Fluorescence spectrometer: A device that can provide an excitation wavelength of 450 nm and an emission wavelength of 510 nm. 2. Steps: 1) Solution preparation: Prepare Pyranine solution and prepare solutions of different concentrations containing Cu+ ions. 2) Reaction: Add Pyranine solution to the solution containing Cu+ ions and mix thoroughly. 3) Fluorescence measurement: Use a fluorescence spectrometer to measure the fluorescence intensity at an excitation wavelength of 450 nm and an emission wavelength of 510 nm. 4) Data analysis: By measuring the fluorescence changes of Cu+ ions at different concentrations, the response of Pyranine in this environment is analyzed for quantitative analysis of Cu+ ion concentration. 2. pH sensitivity analysis 1. Material preparation: 1) Pyranine solution: As mentioned above, prepare a Pyranine solution of appropriate concentration. 2) pH buffer: Prepare buffers of different pH. 2. Steps: 1) Solution preparation: Prepare Pyranine solution and prepare buffers of different pH. 2) Measure pH changes: Add Pyranine solution to buffers of different pH and record its fluorescence intensity. 3) Fluorescence analysis: Analyze the response of Pyranine to pH based on the changes in fluorescence intensity. |
| Synonyms | Solvent Green 7, HPTS |
| Molecular Weight | 524.39 |
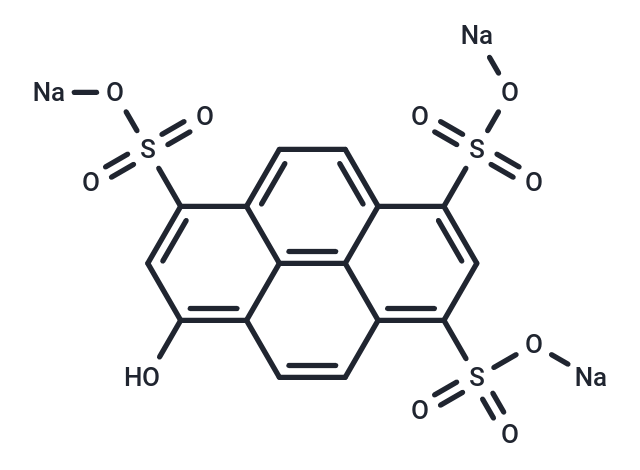
| Formula | C16H7Na3O10S3 |
| Cas No. | 6358-69-6 |
| Smiles | O=S(C1=C(C2=C34)C=CC4=C(O)C=C(S(=O)(O[Na])=O)C3=CC=C2C(S(=O)(O[Na])=O)=C1)(O[Na])=O |
| Relative Density. | 2.15 |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 100 mg/mL (190.7 mM), Sonication is recommended. DMSO: 6.88 mg/mL (13.12 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
DMSO/H2O
H2O
| |||||||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.