 Your shopping cart is currently empty
Your shopping cart is currently empty

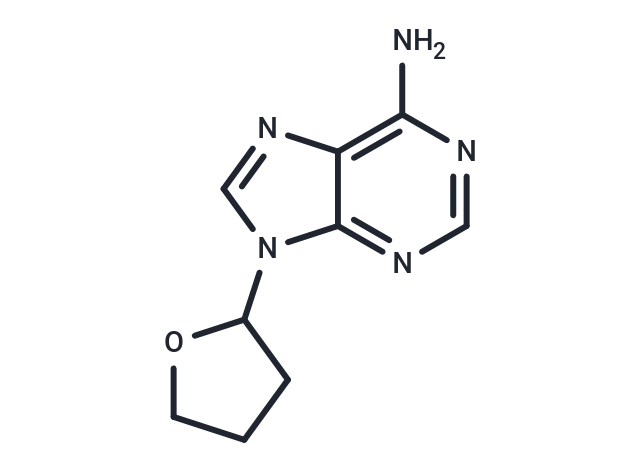
SQ22536 (9-(tetrahydrofuran-2-yl)-9h-purin-6-amine) , the adenosine analogue 9-(Tetrahydro-2-furyl)adenine, inhibited adenylate cyclase activity of crude membrane preparations from catfish (Ictalurus melas) and rat isolated hepatocytes in a non-competitive manner.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 mg | $30 | In Stock | In Stock | |
| 5 mg | $47 | In Stock | In Stock | |
| 10 mg | $72 | In Stock | In Stock | |
| 25 mg | $163 | In Stock | In Stock | |
| 50 mg | $289 | In Stock | In Stock | |
| 100 mg | $433 | In Stock | In Stock | |
| 200 mg | $648 | In Stock | - | |
| 1 mL x 10 mM (in DMSO) | $52 | In Stock | In Stock |
| Description | SQ22536 (9-(tetrahydrofuran-2-yl)-9h-purin-6-amine) , the adenosine analogue 9-(Tetrahydro-2-furyl)adenine, inhibited adenylate cyclase activity of crude membrane preparations from catfish (Ictalurus melas) and rat isolated hepatocytes in a non-competitive manner. |
| In vitro | SQ22536(250 μMol/L) attenuates the inhibitory effect of adenosine against ADP-induced platelet aggregation from 8±5 to 57±5%, respectively (p<0.001). SQ22536 also attenuates an increase of intraplatelet levels of cAMP by adenosine from 29±2 to 9±1 pmol/108 platelets (p<0.05). It has no effect on the platelet antiaggregant activity of inosine (1 to 4 mmol/L) and ADP-induced platelet aggregation[4]. |
| In vivo | SQ22536 abolishes the renal protective effects of liraglutide in KK/Ta-Akita mice. the amelioration of glomerular histopathological damage by liraglutide is eliminated in KK/Ta-Akita mice treated with liraglutide in combination with SQ22536. Renal cAMP does not increase after treatment with SQ22536. In a word, the beneficial actions of liraglutide for treatment of nephropathy are inhibited by the adenylate cyclase inhibitor SQ22536[5]. |
| Cell Research | HMC-1 cells and hCBMCs are plated in 48-well plates and serum-starved overnight. The next day, cells are preincubated with SQ22536 at the indicated concentrations for 30 min before stimulation with CRH (100 nM for HMC-1 or 1 μM for hCBMC) for 3 min in the presence or absence of SQ22536 in serum-free culture media. Cell lysates are then prepared and assayed for protein kinase A activity using ELISA.(Only for Reference) |
| Synonyms | SQ 22536, 9-(tetrahydrofuran-2-yl)-9h-purin-6-amine |
| Molecular Weight | 205.22 |
| Formula | C9H11N5O |
| Cas No. | 17318-31-9 |
| Smiles | Nc1ncnc2n(cnc12)C1CCCO1 |
| Relative Density. | 1.7 g/cm3 |
| Storage | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 10.3 mg/mL (50.19 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (9.75 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.