Powder: -20°C for 3 years | In solvent: -80°C for 1 year


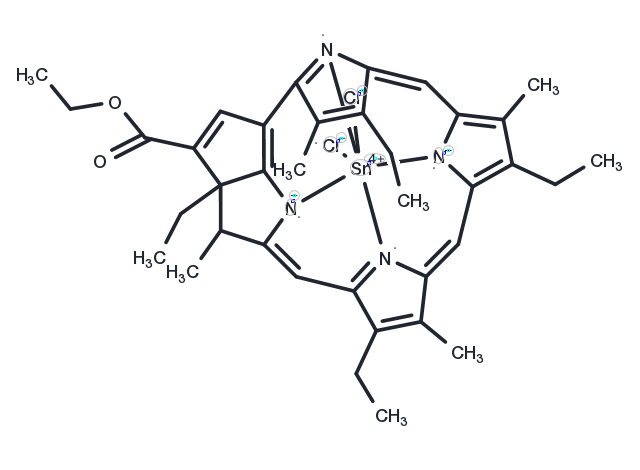
Rostaporfin (also known as REM-001, tin ethyl etiopurpurin, Sn(IV) etiopurpurin, Purlytin, SnET2), is a synthetic purpurin with photosensitizing activity. Rostaporfin preferentially accumulates in tumor cells due to an increased rate of metabolism. Upon exposure to a light source, this agent absorbs light, forming an extended high energy conformational state that produces high quantum yields of singlet oxygen with local cytotoxic effects.
| Pack Size | Availability | Price/USD | Quantity |
|---|---|---|---|
| 25 mg | 6-8 weeks | $ 1,520.00 |
| Description | Rostaporfin (also known as REM-001, tin ethyl etiopurpurin, Sn(IV) etiopurpurin, Purlytin, SnET2), is a synthetic purpurin with photosensitizing activity. Rostaporfin preferentially accumulates in tumor cells due to an increased rate of metabolism. Upon exposure to a light source, this agent absorbs light, forming an extended high energy conformational state that produces high quantum yields of singlet oxygen with local cytotoxic effects. |
| Synonyms | SnET2, tin etiopurpurin dichloride, tin ethyl etiopurpurin dichloride, Purlytin, Sn(IV) etiopurpurin |
| Molecular Weight | 764.37 |
| Formula | C37H42Cl2N4O2Sn |
| CAS No. | 284041-10-7 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
You can also refer to dose conversion for different animals. More
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
Rostaporfin 284041-10-7 tin ethyl etiopurpurin Dichloride tin etiopurpurin Dichloride SnET2 tin etiopurpurin dichloride tin ethyl etiopurpurin dichloride tin etiopurpurin Purlytin tin ethyl etiopurpurin Sn(IV) etiopurpurin inhibitor inhibit
