Powder: -20°C for 3 years | In solvent: -80°C for 1 year


Efaproxiral is a synthetic small molecule with radiosensitizing activity. Efaproxiral increases oxygen levels in hypoxic tumor tissues by binding non-covalently to the hemoglobin tetramer and decreasing hemoglobin-oxygen binding affinity, increasing tumor oxygenation reduces tumor radioresistance.
| Pack Size | Availability | Price/USD | Quantity |
|---|---|---|---|
| 10 mg | 5 days | $ 30.00 | |
| 25 mg | 5 days | $ 46.00 | |
| 50 mg | 5 days | $ 83.00 | |
| 1 mL * 10 mM (in DMSO) | 5 days | $ 74.00 |
| Description | Efaproxiral is a synthetic small molecule with radiosensitizing activity. Efaproxiral increases oxygen levels in hypoxic tumor tissues by binding non-covalently to the hemoglobin tetramer and decreasing hemoglobin-oxygen binding affinity, increasing tumor oxygenation reduces tumor radioresistance. |
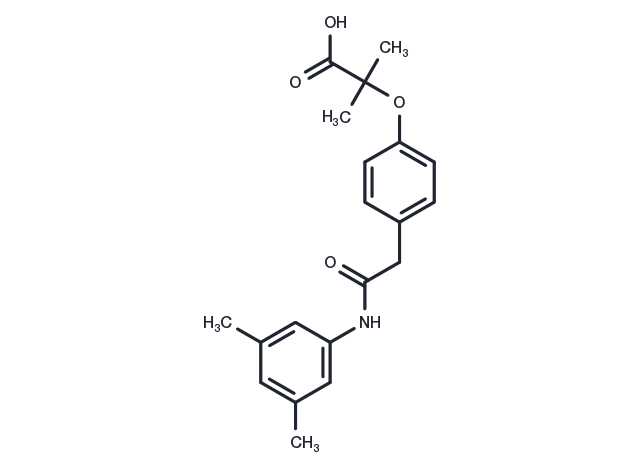
| Synonyms | RSR13, Efaproxyn, RSR 13, RSR-13 |
| Molecular Weight | 341.4 |
| Formula | C20H23NO4 |
| CAS No. | 131179-95-8 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
H2O: Insoluble
DMSO: Soluble
You can also refer to dose conversion for different animals. More
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
Efaproxiral 131179-95-8 RSR13 Efaproxyn RSR 13 RSR-13 inhibitor inhibit
