Powder: -20°C for 3 years | In solvent: -80°C for 1 year


Bismuth(III) oxide is a common starting point for bismuth chemistry. It is usually obtained as a by-product of the smelting of copper and lead ores. Bismuth trioxide is commonly used as a replacement of red lead to produce the “Dragon's eggs” effect in fireworks.
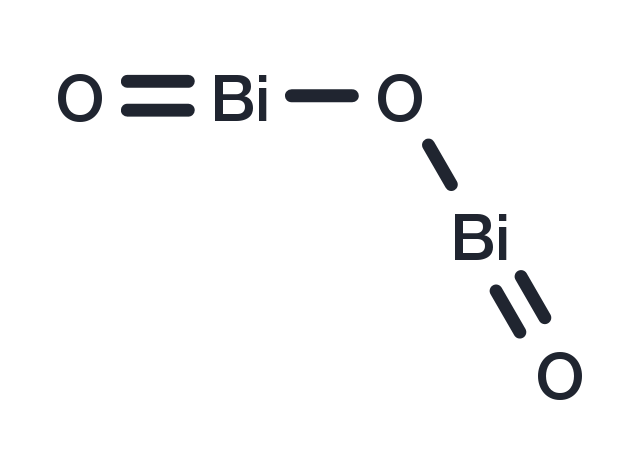
| Pack Size | Availability | Price/USD | Quantity |
|---|---|---|---|
| 5 mg | Inquiry | $ 50.00 |
| Description | Bismuth(III) oxide is a common starting point for bismuth chemistry. It is usually obtained as a by-product of the smelting of copper and lead ores. Bismuth trioxide is commonly used as a replacement of red lead to produce the “Dragon's eggs” effect in fireworks. |
| Synonyms | Bismuth sesquioxide, Bismuth trioxide, Bismutum-oxydatum, Bismuthous oxide |
| Molecular Weight | 465.958 |
| Formula | Bi2O3 |
| CAS No. | 1304-76-3 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
DMSO: Soluble
You can also refer to dose conversion for different animals. More
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
Bismuth oxide 1304-76-3 Bismuth Bismuth sesquioxide Bismuth trioxide Bismutum-oxydatum Bismuth Trioxide Bismuthous Oxide Bismuthous oxide Bismuth Oxide inhibitor inhibit