 Your shopping cart is currently empty
Your shopping cart is currently empty

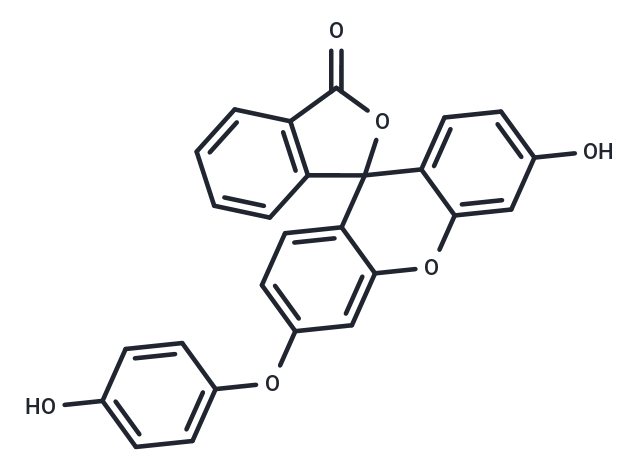
Hydroxyphenyl Fluorescein (HPF) is a cell-permeable fluorescent dye for the detection of highly reactive oxygen species with little or no intrinsic fluorescence.Hydroxyphenyl Fluorescein can be used in fluorescent enzyme labeling and flow cytometry, and can be used to Neurodegeneration and aging. Fluorescence wavelength: Ex/Em = 490/515 nm.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 500 μg | $331 | 35 days | 35 days | |
| 1 mg | $497 | 35 days | 35 days | |
| 5 mg | Inquiry | 35 days | 35 days |
| Description | Hydroxyphenyl Fluorescein (HPF) is a cell-permeable fluorescent dye for the detection of highly reactive oxygen species with little or no intrinsic fluorescence.Hydroxyphenyl Fluorescein can be used in fluorescent enzyme labeling and flow cytometry, and can be used to Neurodegeneration and aging. Fluorescence wavelength: Ex/Em = 490/515 nm. |
| Cell Research | Operation steps: a. Preparation of stock solution: Prepare 10 mM HPF with DMSO; (it is recommended to store at -20 ℃ or -80 ℃ in the dark after aliquoting) b. Preparation of HPF working solution Dilute the stock solution and dissolve it in serum-free cell culture medium or PBS to prepare 1-10 μM HPF working solution; (select the appropriate working solution concentration according to experimental requirements and prepare it for immediate use) c. Cell staining 1. Suspended cells (6-well plate) a. Centrifuge at 1000 g for 3-5 minutes at 4℃, and then discard the supernatant. Wash twice with PBS for 5 minutes each. The cell density is 1×106/mL; b. Add 1 mL of working solution and incubate at room temperature for 15 minutes; c. Centrifuge at 4℃ and 400 g for 3-4 minutes, and discard the supernatant; d. Wash twice with PBS, 5 minutes each time; e. Resuspend cells with serum-free cell culture medium or PBS. Observe with fluorescence microscope or flow cytometer. 2. Adherent cells a. Culture adherent cells on sterile coverslips; b. Remove coverslips from culture medium and aspirate excess culture medium; c. Add 100 μL working solution, shake gently to completely cover cells, and incubate at room temperature for 15 minutes; d. Wash twice with culture medium, 5 minutes each time. Observe with fluorescence microscope or flow cytometer. The above information is based on published literature. Experimental procedures should be appropriately modified to meet specific research demands. |
| Synonyms | HPF, 3'-p-(Hydroxyphenyl) fluorescein |
| Molecular Weight | 424.4 |
| Formula | C26H16O6 |
| Cas No. | 359010-69-8 |
| Smiles | O=C1OC2(C3=CC=C(O)C=C3OC4=CC(OC5=CC=C(O)C=C5)=CC=C42)C=6C=CC=CC16 |
| Storage | store at low temperature,keep away from direct sunlight | store at -20°C | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||
| Solubility Information | DMSO: 10 mg/mL (23.56 mM), Sonication is recommended. DMF: 8 mg/mL (18.85 mM), Sonication is recommended. Ethanol: 8 mg/mL (18.85 mM), Sonication is recommended. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMF/Ethanol/DMSO
| |||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.