 Your shopping cart is currently empty
Your shopping cart is currently empty

Sunvozertinib (DZD9008) is a potent ErbBs and BTK inhibitor, with inhibitory effects on EGFR, Her2, and mutant epidermal growth factor. Sunvozertinib (DZD9008) showed inhibitory effects on EGFR exon 20 NPH insertion, EGFR exon 20 ASV insertion, EGFR L858R and T790M mutations, as well as Her2 exon 20 YVMA and EGFR WT A431 with IC50 of 20.4, 20.4, 1.1, 7.5, and 80.4 nM.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $30 | In Stock | In Stock | |
| 5 mg | $58 | In Stock | In Stock | |
| 10 mg | $82 | In Stock | In Stock | |
| 25 mg | $133 | In Stock | In Stock | |
| 50 mg | $223 | In Stock | In Stock | |
| 100 mg | $392 | In Stock | In Stock | |
| 500 mg | $1,460 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $162 | In Stock | In Stock |
| Description | Sunvozertinib (DZD9008) is a potent ErbBs and BTK inhibitor, with inhibitory effects on EGFR, Her2, and mutant epidermal growth factor. Sunvozertinib (DZD9008) showed inhibitory effects on EGFR exon 20 NPH insertion, EGFR exon 20 ASV insertion, EGFR L858R and T790M mutations, as well as Her2 exon 20 YVMA and EGFR WT A431 with IC50 of 20.4, 20.4, 1.1, 7.5, and 80.4 nM. |
| Targets&IC50 | EGFR (L858R/T790M):1.1 nM, HER2 exon20 YVMA:7.5 nM, EGFR (exon 20 insertion):20.4 nM |
| In vitro | METHODS: To test the cellular activity of Sunvozertinib (DZD9008) , 14 different EGFRexon20ins were engineered into the Ba/F3 cell line. RESULTS In these cell lines, Sunvozertinib (DZD9008) showed potent pEGFR downregulation activity with IC50 ranging from 6 to 40 nM; in other cell lines expressing EGFR sensitive mutations, T790M resistance mutations, and rare mutations, Sunvozertinib (DZD9008) downregulated pEGFR more effectively with IC50 ranging from 1.1 to 12 nM; in the A431 cell line overexpressing wild-type EGFR, Sunvozertinib (DZD9008) was less potent in downregulating pEGFR with an IC50 of 58 nM. [1] |
| In vivo | METHODS: Blood samples from the LU3075 PDX mouse model were collected at 0.5, 1, 2, 4, 8, and 24 hours after administration of sunvozertinib (DZD9008) (12.5, 25, and 50 mg/kg, orally, twice daily) on day 28 for LC/MS-MS analysis. RESULTS Oral sunvozertinib (DZD9008) showed profound anti-tumor efficacy in a dose-dependent manner. [2] |
| Synonyms | DZD-9008, DZD9008, DZD 9008 |
| Molecular Weight | 584.08 |
| Formula | C29H35ClFN7O3 |
| Cas No. | 2370013-12-8 |
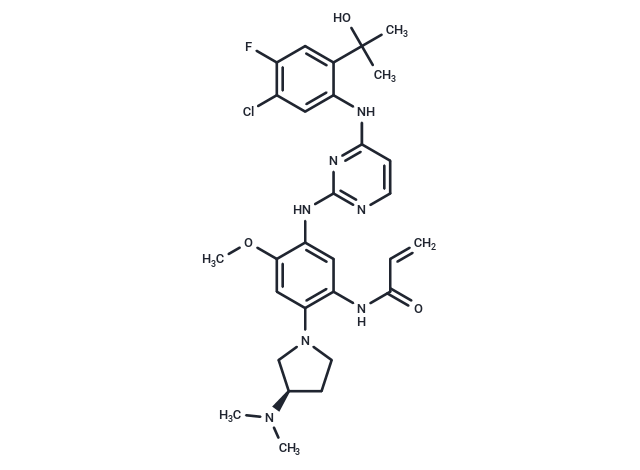
| Smiles | COc1cc(N2CC[C@H](C2)N(C)C)c(NC(=O)C=C)cc1Nc1nccc(Nc2cc(Cl)c(F)cc2C(C)(C)O)n1 |
| Storage | keep away from direct sunlight,store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 247.5 mg/mL (423.74 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5 mg/mL (8.56 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.