Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
BHPI is a potent inhibitor of nuclear estrogen receptor ERα–regulated gene expression that induces sustained ERα-dependent activation of the endoplasmic reticulum stress sensor and unfolded protein response, leading to persistent inhibition of protein synthesis, and in ERα-positive cancer cells it hyperactivates plasma membrane PLCγ to generate IP3, deplete ER calcium stores via IP3R channels, and convert the normally protective UPR into a toxic response, making BHPI a valuable probe for estrogen signaling and ER stress–based cancer research.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 mg | $89 | 6-8 weeks | 6-8 weeks | |
| 50 mg | $755 | 6-8 weeks | 6-8 weeks | |
| 100 mg | $1,240 | 6-8 weeks | 6-8 weeks | |
| 1 mL x 10 mM (in DMSO) | $197 | 6-8 weeks | 6-8 weeks |
| Description | BHPI is a potent inhibitor of nuclear estrogen receptor ERα–regulated gene expression that induces sustained ERα-dependent activation of the endoplasmic reticulum stress sensor and unfolded protein response, leading to persistent inhibition of protein synthesis, and in ERα-positive cancer cells it hyperactivates plasma membrane PLCγ to generate IP3, deplete ER calcium stores via IP3R channels, and convert the normally protective UPR into a toxic response, making BHPI a valuable probe for estrogen signaling and ER stress–based cancer research. |
| In vitro | In cellular assays, BHPI inhibited the proliferation of ERα-positive breast, endometrial, and ovarian cancer cells at concentrations between 100 and 1000 nM. It maintained activity (25 nM) in antiestrogen-resistant cell lines harboring ERα mutations (Y537S, D538G) by triggering UPR hyperactivation, protein synthesis inhibition, and apoptosis [1]. |
| In vivo | In MCF-7 breast cancer xenograft models, intraperitoneal (i.p.) administration of BHPI (10-15 mg/kg) resulted in tumor regression. When administered daily for 10 days or every other day, tumor regression was observed in 48 out of 52 treated tumors. This effect is attributed to the sustained activation of UPR stress within the tumor tissue [1]. |
| Molecular Weight | 331.36 |
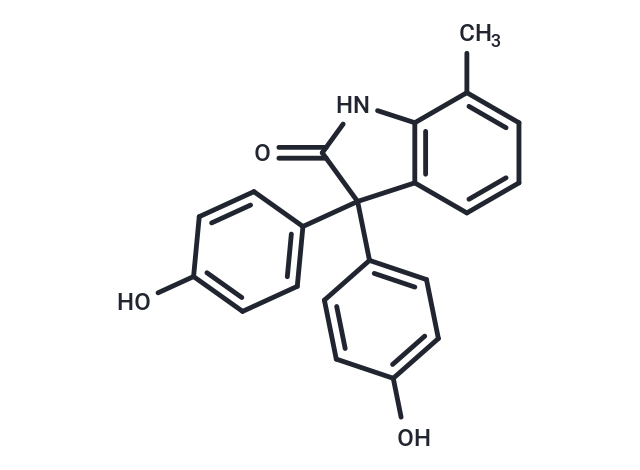
| Formula | C21H17NO3 |
| Cas No. | 56632-39-4 |
| Smiles | O=C1NC=2C(=CC=CC2C1(C3=CC=C(O)C=C3)C4=CC=C(O)C=C4)C |
| Relative Density. | 1.320 g/cm3 (Predicted) |
| Storage | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 100 mg/mL (301.79 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 4 mg/mL (12.07 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.