Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Scutellarin (Scutellarein-7-glucuronide), an active flavone isolated from Scutellaria baicalensis, can inhibit RANKL-mediated MAPK and NF-κB signaling pathway in osteoclasts, and down-regulate the STAT3/Girdin/Akt signaling in HCC cells.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 mg | $30 | In Stock | In Stock | |
| 10 mg | $41 | In Stock | In Stock | |
| 25 mg | $68 | In Stock | In Stock | |
| 50 mg | $121 | In Stock | In Stock | |
| 100 mg | $155 | In Stock | In Stock | |
| 500 mg | $377 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $46 | In Stock | In Stock |
| Description | Scutellarin (Scutellarein-7-glucuronide), an active flavone isolated from Scutellaria baicalensis, can inhibit RANKL-mediated MAPK and NF-κB signaling pathway in osteoclasts, and down-regulate the STAT3/Girdin/Akt signaling in HCC cells. |
| Targets&IC50 | K562 cells viability:6 μM, 4-acetamidophenol:108.20 ± 0.657 µM, Rosuvastatin uptake (mediated via hOATP1B3):45.54 ± 6.67 μM, CYP1A2:95.2 µM (Ki), Rosuvastatin uptake (mediated via rOATP1B2):27.58 ± 3.97 μM |
| In vitro | Scutellarin significantly lowers HepG2 cell viability in a dose-dependent manner and hampers HCC cells' migration and invasion in vitro. It notably decreases STAT3 and Girdin expression, along with the phosphorylation of STAT3 and Akt in HCC cells. The overexpression of STAT3 reverses the suppression by scutellarin of Girdin expression and Akt activation, restoring HCC cells' ability to migrate and invade. Furthermore, overexpression of Girdin nullifies scutellarin's inhibitory effects on Akt phosphorylation, and on the migration and invasion of HCC cells. Scutellarin can prevent HCC cell metastasis in vivo and restrict their migration and invasion in vitro by targeting the STAT3/Girdin/Akt pathway. Additionally, scutellarin has a neuroprotective role by inhibiting microglial activation, mitigating neuroinflammation, and promoting astrocytic response by upregulating neurotrophic factor expression. This demonstrates a beneficial interaction between activated microglia and astrocytes. Scutellarin also impedes RANKL-mediated osteoclastogenesis and bone resorption by reducing osteoclast-specific gene expression (TRAP, cathepsin K, c-Fos, NFATc1) and inhibiting the RANKL-mediated MAPK and NF-κB signaling pathways, including JNK1/2, p38, ERK1/2, and IκBα phosphorylation. |
| In vivo | Scutellarin administration at a dosage of 50 mg/kg/day notably reduces both lung and intrahepatic metastasis of HCC tumors in vivo. Comparatively, the scutellarin-treated group exhibits a significant decrease in the number of metastatic tumors in the lung and liver versus the control group[1]. Furthermore, rats receiving Scutellarin show marked improvement in neurobehavioral deficits in contrast to the SAH group, alongside an enhancement in eNOS expression[4]. |
| Cell Research | HepG2 cells (1×105/well) are cultured in 96-well plates and treated in triplicate with scutellarin at concentrations of 5, 10, 20, 30, and 100 μM or vehicle alone for 24 h. The cellular viability is tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and is expressed as a percentage of proliferation versus controls. |
| Synonyms | Scutellarein-7-glucuronide, Breviscapinun, Breviscapine, Breviscapin |
| Molecular Weight | 462.36 |
| Formula | C21H18O12 |
| Cas No. | 27740-01-8 |
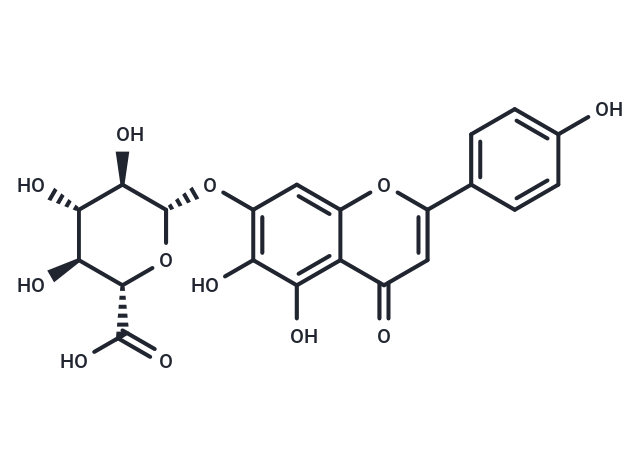
| Smiles | O[C@H]1[C@H](Oc2cc3oc(cc(=O)c3c(O)c2O)-c2ccc(O)cc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |
| Relative Density. | 1.81 g/cm3 |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 240 mg/mL (519.08 mM), Sonication is recommended. H2O: insoluble | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.