 Your shopping cart is currently empty
Your shopping cart is currently empty

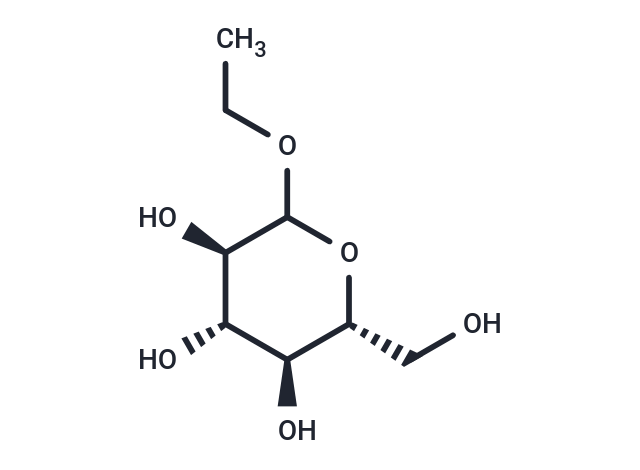
Ethyl glucoside (β-D-Glucopyranoside, ethyl), a shale inhibitor for water-based drilling fluids, is a versatile initiator of enzyme-catalyzed regioselective lactone ring-opening polymerization and is a natural compound found in Sisyrinchium palmifolium.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 10 mg | $30 | In Stock | In Stock | |
| 25 mg | $53 | In Stock | In Stock | |
| 50 mg | $81 | In Stock | In Stock |
| Description | Ethyl glucoside (β-D-Glucopyranoside, ethyl), a shale inhibitor for water-based drilling fluids, is a versatile initiator of enzyme-catalyzed regioselective lactone ring-opening polymerization and is a natural compound found in Sisyrinchium palmifolium. |
| In vitro | EG exists as alpha (α) and beta (β) isomers. In this study, 0.48 μM of α-EG was found to increase the proliferation of normal human dermal fibroblasts (NHDF) by 121.0%, and the amount of collagen I produced by NHDF increased by 159.6% at an α-EG concentration of 0.048 μM, compared to those in cells cultured without α-EG. In NHDF cultured in α-EG-supplemented medium, the expression of fibroblast growth factor I and VII mRNA increased by 148.8 and 153.1%, at an α-EG concentration of 4.8 and 0.048 μM, respectively, as measured by a quantitative reverse transcription-polymerase chain reaction. Transcript levels of type I collagen genes, COL1A1 and COL1A2, increased by 152.4 and 129.7%, respectively, and that of a type III collagen gene, COL3A1, increased by 131.8% at an α-EG concentration of 0.48 μM.[3][4] |
| In vivo | Metabonomic screening of human urine samples using 1H NMR spectroscopy has revealed the presence of signals resulting from the excretion of Ethyl glucoside. Experiments in volunteers have demonstrated that this Ethyl glucoside results from dietary exposure to the compound, which is present in beverages such as rice wine and sake, rather than representing a new route for the metabolism of ethanol by humans. The limited studies undertaken in volunteers indicate that Ethyl glucoside has a longer biological half life than ethanol itself. The potential problems associated with using this glucoside metabolite as a marker of ethanol consumption are considered.[2] |
| Synonyms | β-D-Glucopyranoside, ethyl, Ethyl D-glucoside |
| Molecular Weight | 208.21 |
| Formula | C8H16O6 |
| Cas No. | 3198-49-0 |
| Smiles | CCOC1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |
| Relative Density. | 1.40 g/cm3 |
| Storage | Pure form: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 60 mg/mL (288.17 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (9.61 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.