 Your shopping cart is currently empty
Your shopping cart is currently empty

AZD7687 is a potent and selective DGAT1 inhibitor with an IC50 value of 80 nM (hDGAT1). IC50 value: 80 nM [1] Target: DGAT1 in vitro: Plasma AZD7687 exposure was measured repeatedly. AZD7687 markedly reduced postprandial TAG excursion with a steep concentration-effect relationship [2]. Postprandial serum TAG excursion was measured during 8 h after a standardized mixed meal with fat energy content of 60% (SMM 60%; five cohorts, 1-20 mg), before (baseline) and after dosing, to assess effects on gut DGAT1 activity. in vivo: Multiple doses of AZD7687 (1, 2.5, 5, 10 and 20 mg/day, n=6 or n=12 for each) or placebo (n=20) were administered for 1 week. With AZD7687 doses >5 mg/day, gastrointestinal (GI) side effects increased; 11/18 of these participants discontinued treatment owing to diarrhoea[3]. Dose-dependent reductions in postprandial serum TAG were demonstrated with AZD7687 doses ≥5mg compared with placebo (p<0.01). Significant (p<0.001) increases in plasma GLP-1 and PYY levels were seen at these doses, but no clear effect on gastric emptying was demonstrated at the end of treatment.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 25 mg | $862 | 8-10 weeks | 8-10 weeks | |
| 50 mg | $1,120 | 8-10 weeks | 8-10 weeks | |
| 100 mg | $1,690 | 8-10 weeks | 8-10 weeks |
| Description | AZD7687 is a potent and selective DGAT1 inhibitor with an IC50 value of 80 nM (hDGAT1). IC50 value: 80 nM [1] Target: DGAT1 in vitro: Plasma AZD7687 exposure was measured repeatedly. AZD7687 markedly reduced postprandial TAG excursion with a steep concentration-effect relationship [2]. Postprandial serum TAG excursion was measured during 8 h after a standardized mixed meal with fat energy content of 60% (SMM 60%; five cohorts, 1-20 mg), before (baseline) and after dosing, to assess effects on gut DGAT1 activity. in vivo: Multiple doses of AZD7687 (1, 2.5, 5, 10 and 20 mg/day, n=6 or n=12 for each) or placebo (n=20) were administered for 1 week. With AZD7687 doses >5 mg/day, gastrointestinal (GI) side effects increased; 11/18 of these participants discontinued treatment owing to diarrhoea[3]. Dose-dependent reductions in postprandial serum TAG were demonstrated with AZD7687 doses ≥5mg compared with placebo (p<0.01). Significant (p<0.001) increases in plasma GLP-1 and PYY levels were seen at these doses, but no clear effect on gastric emptying was demonstrated at the end of treatment. |
| Molecular Weight | 367.44 |
| Formula | C21H25N3O3 |
| Cas No. | 1166827-44-6 |
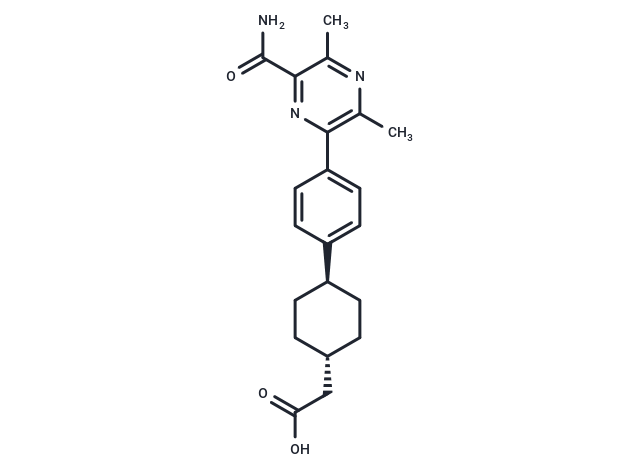
| Smiles | Cc1nc(C)c(nc1C(N)=O)-c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |
| Relative Density. | 1.201 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (136.08 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+90% Saline: 2.5 mg/mL (6.8 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.