Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Ammonium chloride is a compound commonly used in molecular biology research. As a dipolar compound that regulates pH, it can induce intracellular alkalinization and metabolic acidosis, thereby affecting enzyme activity and biological processes. Additionally, ammonium chloride acts as an inhibitor of autophagy and lysosomal function, and can be used to establish kidney stone models.
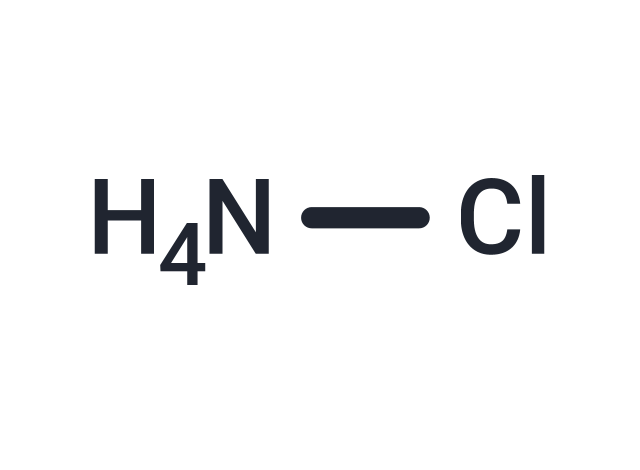
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 200 mg | $35 | In Stock | In Stock | |
| 500 mg | $55 | In Stock | In Stock | |
| 1 g | $80 | In Stock | In Stock | |
| 5 g | $139 | - | In Stock | |
| 10 g | $172 | - | In Stock | |
| 25 g | $288 | - | In Stock |
| Description | Ammonium chloride is a compound commonly used in molecular biology research. As a dipolar compound that regulates pH, it can induce intracellular alkalinization and metabolic acidosis, thereby affecting enzyme activity and biological processes. Additionally, ammonium chloride acts as an inhibitor of autophagy and lysosomal function, and can be used to establish kidney stone models. |
| In vitro | Ammonium Chloride (NH4Cl) reduces the yield of reovirus during infection of mouse L cells.[2] |
| In vivo | Ammonium Chloride (0.28 M in drinking water; 8-9-week-old C57B/L6 mice) promotes the survival of myocardial cells in vivo by decreasing contractile dysfunction, cardiac hypertrophy, inflammation, apoptosis and autophagy. Ammonium Chloride effectively improved doxorubicin (DOX)-induced cardiomyocyte apoptosis and cardiac dysfunction in mice.[1] |
| Molecular Weight | 53.49 |
| Formula | ClH4N |
| Cas No. | 12125-02-9 |
| Smiles | Cl[NH4] |
| Storage | In solvent: -80°C for 1 year | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 52 mg/mL (972.14 mM), Sonication is recommended. H2O: 80 mg/mL (1495.61 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 2.5 mg/mL (46.74 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO/H2O
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.