Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Nicaraven, a hydroxyl radical scavenger, has neuroprotective and antivasospastic effects.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $29 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock |
| Description | Nicaraven, a hydroxyl radical scavenger, has neuroprotective and antivasospastic effects. |
| In vitro | Nicaraven causes a dose-dependent, slight inhibition of poly (ADP-ribose) synthetase activation, possibly due to a direct inhibitory effect on the catalytic activity of poly (ADP-ribose) synthetase in RAW murine macrophages stimulated with peroxynitrite. Nicaraven partially protects against the peroxynitrite-induced suppression of mitochondrial respiration in RAW macrophages and causes a slight, dose-dependent inhibition of nitrite production in RAW macrophages stimulated with bacterial lipopolysaccharide. [1] Nicaraven (0.35 mM) significantly inhibits the maximum aggregation rate induced by adenosine diphosphate (ADP) in the healthy volunteer platelets. Nicaraven (1.75 mM) significantly reduces the maximum aggregation rate induced by collagen in platelets. Nicaraven induces dose-dependent inhibition of platelet aggregation in both healthy volunteers and patients with cerebral thrombosis. [2] |
| In vivo | Nicaraven inhibits lipid peroxidation in the liver of beagle dogs, improves hepatic and systemic hemodynamics and energy metabolism, and suppresses liver enzyme release, endothelin-1 elevation in hepatic venous blood, histologic damage, and neutrophil infiltration into the liver [3]. Nicaraven (20 mg/kg) slightly reduces infarction volume in male Sprague-Dawley rats subjected to transient focal ischemia, while a dose of 60 mg/kg significantly reduces infarction volume by 18.6% and 20.9% in pre- and posttreatment groups, respectively [4]. |
| Molecular Weight | 284.31 |
| Formula | C15H16N4O2 |
| Cas No. | 79455-30-4 |
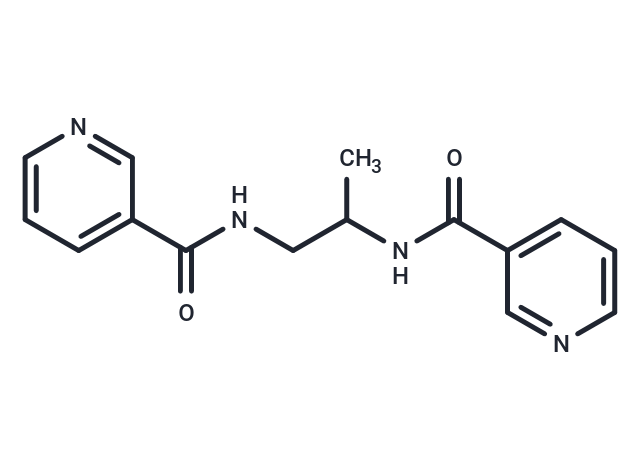
| Smiles | CC(CNC(=O)c1cccnc1)NC(=O)c1cccnc1 |
| Relative Density. | 1.213 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | Ethanol: 53 mg/mL (186.42 mM), Sonication is recommended. H2O: 52 mg/mL (182.9 mM), Sonication is recommended. DMSO: 45 mg/mL (158.28 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO/H2O/Ethanol
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.