 Your shopping cart is currently empty
Your shopping cart is currently empty

Erastin is an iron death activator that acts on the mitochondrial VDAC in a ROS- and iron-dependent manner. Erastin has anti-tumor activity and acts selectively on tumor cells with RAS-carcinogenic mutations.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $41 | In Stock | In Stock | |
| 1 mg * 2 | $57 | In Stock | In Stock | |
| 1 mg * 5 | $89 | In Stock | In Stock | |
| 1 mg * 10 | $129 | In Stock | In Stock | |
| 1 mg * 25 | $213 | In Stock | In Stock | |
| 1 mg * 50 | $316 | In Stock | In Stock | |
| 1 mg * 100 | $468 | In Stock | In Stock | |
| 1 mg * 200 | $687 | In Stock | In Stock |
| Description | Erastin is an iron death activator that acts on the mitochondrial VDAC in a ROS- and iron-dependent manner. Erastin has anti-tumor activity and acts selectively on tumor cells with RAS-carcinogenic mutations. |
| Targets&IC50 | HT1080 cells:10 μM, HepG2 cells:5 μM, U-87MG cells:2 - 3 μM |
| In vitro | METHODS: Human gastric cancer cells HGC-27 were treated with Erastin (1-50 μM) for 24 h, and cell growth inhibition was detected by CCK-8. RESULTS: Erastin dose-dependently inhibited HGC-27 cell growth with an IC50 of approximately 14.39 μM. [1] METHODS: Human melanoma cells A375 were treated with Erastin (2-10 μM) for 3-12 h. The expression levels of target proteins were detected by Western Blot. RESULTS: Erastin treatment caused a significant down-regulation of VDAC2 and VDAC3, and a slight decrease of VDAC1 in A375 cells. [2] METHODS: Human colon cancer cells HT-29 were treated with Erastin (0.1-30 μM) for 12 h. The intracellular ROS levels were measured by Flow Cytometry. RESULTS: Erastin treatment significantly increased ROS levels in HT-29 cells. [3] |
| In vivo | METHODS: To test the antitumor activity in vivo, erastin (20 mg/kg in 20 μL DMSO plus 130 μL corn oil) was intraperitoneally injected into NSG mice bearing human prostate cancer tumors DU145, ARCaP, PC3, or H660 once daily for two to five weeks. RESULTS: Erastin treatment significantly inhibited the growth of human prostate cancer tumors, indicating antitumor activity in vivo. [4] METHODS: To study the effect of erastin treatment on anticancer radiation efficiency, erastin (15 mg/kg in 5% DMSO/corn oil) was injected intraperitoneally into BALB/c Slc-nu/nu mice bearing human lung adenocarcinoma tumor NCI-H1975 once a day for three days. Twenty-four hours after the last erastin injection, the anesthetized mice were locally irradiated with 3 Gy X-rays. RESULTS: Erastin-treated NCI-H1975 cell transplanted mice showed a trend of sensitization to X-ray irradiation with a concomitant decrease in intra-tumoral glutathione concentration. [5] |
| Cell Research | BJeLR cells were plated at 100,000 cells/dish in 35 mm tissue culture dishes. After 12h cells were treated with vehicle (DMSO; 10 hrs), erastin (37 μM; 10 hrs), staurosporine (750 nM; 8 hrs), hydrogen peroxide (16 mM; 1 hr) or rapamycin (100 nM; 24 hrs). Cells were fixed with 2.5% glutaraldehyde in 0.1 M Sorenson's buffer (0.1 M H2PO4, 0.1 M HPO4 (pH 7.2)) for at least 1 h, and then treated with 1% OsO4 in 0.1 M Sorenson's buffer for 1 h. Enblock staining used 1% tannic acid. After dehydration through an ethanol series, cells were embedded in Lx-112 and Embed-812 (EMS). Thin sections were cut on an MT-7000 ultramicrotome, stained with 1% uranyl acetate and 0.4% lead citrate, and examined under a Jeol JEM-1200 EXII electron microscope. Pictures were taken on an ORCA-HR digital camera at 5,000-50,000-fold magnification [1]. |
| Animal Research | Tumor growth studies were performed in severe combined immunodeficient (SCID) mice xenograft model. Briefly, 2×10^6 viable HT-29 cells in 100 μL of growth medium (per mouse) were subcutaneously inoculated, and mice bearing ~100 mm3 tumors were randomly divided into three groups with 10 mice per group. Mice were treated daily with 10 or 30 mg/kg body weight of erastin (intraperitoneal injection, for 4 weeks) or vehicle control (Saline). Tumor volumes were calculated by the modified ellipsoid formula: (π / 6) ×AB2, where A is the longest and B is the shortest perpendicular axis of a tumor mass. Mice body weights were also recorded every week. Humane endpoints were always utilized to minimize mice suffering. Animals were observed on daily bases. Signs such as significant-reduced locomotion, severe diarrhea, severe piloerection or a sudden weight loss (> 20%) were recorded. If animals reached these endpoints they were euthanized by exsanguination under 2,2,2-tribromoethanol anesthesia (4 mg/10 g body weight). All injections were performed under the 2,2,2-tribromoethanol anesthesia method [3]. |
| Molecular Weight | 547.04 |
| Formula | C30H31ClN4O4 |
| Cas No. | 571203-78-6 |
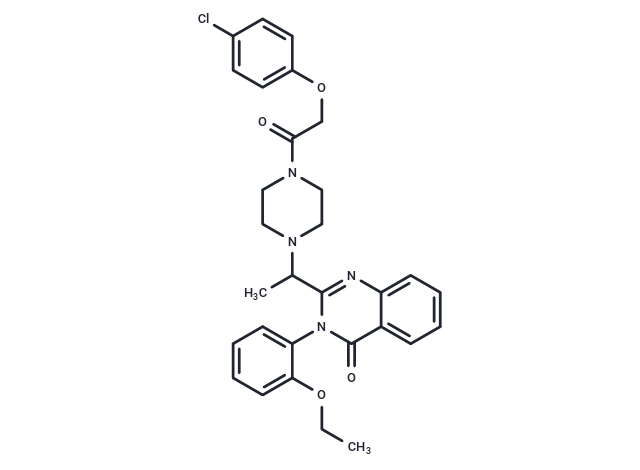
| Smiles | CCOc1ccccc1-n1c(nc2ccccc2c1=O)C(C)N1CCN(CC1)C(=O)COc1ccc(Cl)cc1 |
| Relative Density. | 1.28 g/cm3 (Predicted) |
| Storage | store at low temperature,The compound is unstable in solution. Please use soon | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||
| Solubility Information | DMSO: 25 mg/mL (45.7 mM), Sonication is recommended. The compound is unstable in solution. Please use soon. Ethanol: < 1 mg/mL (insoluble or slightly soluble) H2O: < 1 mg/mL (insoluble or slightly soluble) | |||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 1.6 mg/mL (2.92 mM), Solution.The compound is unstable in solution. Please use soon. 20% Cremophor EL: 10 mg/mL (18.28 mM), Suspension. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.