Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Perflubron (Perfluorooctyl bromide) can be emulsified with egg phospholipids (EYP) and displays exceptionally fast excretion characteristics. Perflubron(1-Bromoheptadecafluorooctane; Heptadecafluorooctyl bromide; Perfluorooctyl bromide) is a contrast medium for magnetic resonance imaging and sonography.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 2 g | $38 | - | In Stock | |
| 5 g | $59 | - | In Stock | |
| 10 g | $86 | - | In Stock | |
| 25 g | $142 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock | In Stock |
| Description | Perflubron (Perfluorooctyl bromide) can be emulsified with egg phospholipids (EYP) and displays exceptionally fast excretion characteristics. Perflubron(1-Bromoheptadecafluorooctane; Heptadecafluorooctyl bromide; Perfluorooctyl bromide) is a contrast medium for magnetic resonance imaging and sonography. |
| Animal Research | I. Magnetic Resonance Imaging (MRI) Application 1. Material Preparation: 1) Perflubron Emulsion: Perflubron is mixed with phosphatidylcholine (EYP) to form a stable emulsion. The concentration of Perflubron in the emulsion is usually 10%-40%. 2) Phosphatidylcholine (EYP): A lipid used to emulsify Perflubron, enhancing its solubility and stability in the aqueous phase. 3) MRI Equipment: Magnetic resonance imaging equipment equipped with high-resolution imaging capabilities. 2. Steps: 1) Preparation: Perflubron is mixed with EYP in a sterile environment to form a stable emulsion, and the concentration is adjusted according to imaging needs. 2) Injection: The emulsion is administered to the patient by intravenous injection, and an MRI scan is performed after the injection. 3) MRI Imaging: An MRI scan is performed and the distribution of the contrast agent is monitored. Perflubron can enhance tissue contrast, especially when monitoring vascular flow or assessing blood volume. 4) After imaging: Perflubron is usually excreted quickly through the lungs, which helps reduce the patient's exposure to contrast agents. 2. Ultrasound examination application 1. Material preparation: 1) Perflubron emulsion: As with magnetic resonance imaging, Perflubron emulsion is emulsified with lecithin to form a microbubble solution suitable for ultrasound imaging. 2) Ultrasound equipment: High-frequency ultrasound equipment that can detect microbubble signals. 2. Steps: 1) Preparation and injection: Prepare Perflubron microbubble emulsion and perform ultrasound examination after intravenous injection. 2) Imaging: Use ultrasound equipment to image blood vessels and tissue structures. Perflubron microbubbles can significantly improve the clarity of blood vessels and tissue boundaries. 3) After imaging: Microbubbles are usually excreted through the lungs, providing a fast, non-invasive imaging method. Precautions: 1) Dosage: The dose should be reasonably selected according to the imaging procedure and the specific situation of the patient to avoid excessive doses that lead to unnecessary exposure. 2) Patient safety: As with all contrast agents, there is a risk of allergic reactions. Patients should be monitored during and after the injection to detect adverse reactions. 3) Excretion monitoring: Although Perflubron is excreted rapidly, its excretion through the lungs must be monitored to avoid respiratory problems. |
| Synonyms | PFOB, Perfluorooctyl bromide |
| Molecular Weight | 498.96 |
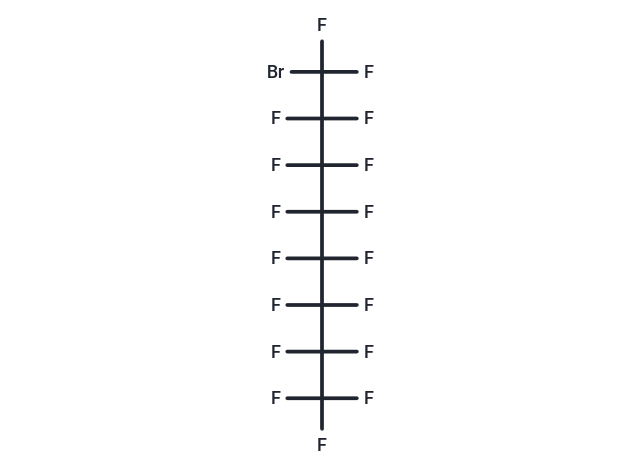
| Formula | C8BrF17 |
| Cas No. | 423-55-2 |
| Smiles | FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)Br |
| Relative Density. | 1.93 g/cm3 at 25℃ (lit.) |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (100.21 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 1.67 mg/mL (3.35 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.