 Your shopping cart is currently empty
Your shopping cart is currently empty

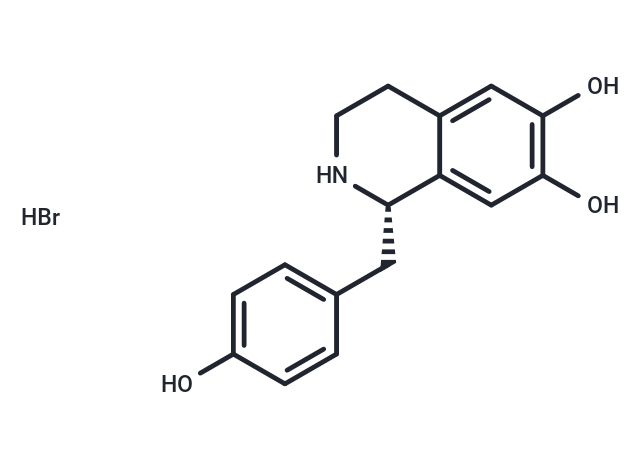
(S)-Higenamine hydrobromide, an S-enantiomer of Higenamine, serves as the preliminary compound in benzylisoquinoline alkaloid biosynthesis. It is formed through the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) via norcoclaurine synthase (NCS)[1].
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $162 | Inquiry | Inquiry |
| Description | (S)-Higenamine hydrobromide, an S-enantiomer of Higenamine, serves as the preliminary compound in benzylisoquinoline alkaloid biosynthesis. It is formed through the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) via norcoclaurine synthase (NCS)[1]. |
| In vitro | The biosynthetic pathway for benzylisoquinoline alkaloids begins with the enzyme-catalyzed condensation of dopamine and 4-hydrophenylacetaldehyde, producing (S)-norcoclaurine. These substrates are secondary metabolites stemming from the decarboxylation, hydroxylation, and deamination of tyrosine[1]. |
| Molecular Weight | 352.22 |
| Formula | C16H18BrNO3 |
| Cas No. | 105990-27-0 |
| Smiles | Br.Oc1ccc(C[C@@H]2NCCc3cc(O)c(O)cc23)cc1 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.