Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) which is under development by Kissei Pharmaceutical and ObsEva for the treatment of uterine fibroids, endometriosis, and adenomyosis. As of December 2020, it is under review for approval for uterine fibroids, is in phase III clinical trials for endometriosis, and is in phase II clinical studies for adenomyosis.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 mg | $35 | In Stock | In Stock | |
| 5 mg | $84 | In Stock | In Stock | |
| 10 mg | $129 | In Stock | In Stock | |
| 25 mg | $283 | In Stock | In Stock | |
| 50 mg | $463 | In Stock | In Stock | |
| 1 mL x 10 mM (in DMSO) | $94 | In Stock | In Stock |
| Description | Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) which is under development by Kissei Pharmaceutical and ObsEva for the treatment of uterine fibroids, endometriosis, and adenomyosis. As of December 2020, it is under review for approval for uterine fibroids, is in phase III clinical trials for endometriosis, and is in phase II clinical studies for adenomyosis. |
| Molecular Weight | 508.42 |
| Formula | C22H15F3N2O7S |
| Cas No. | 935283-04-8 |
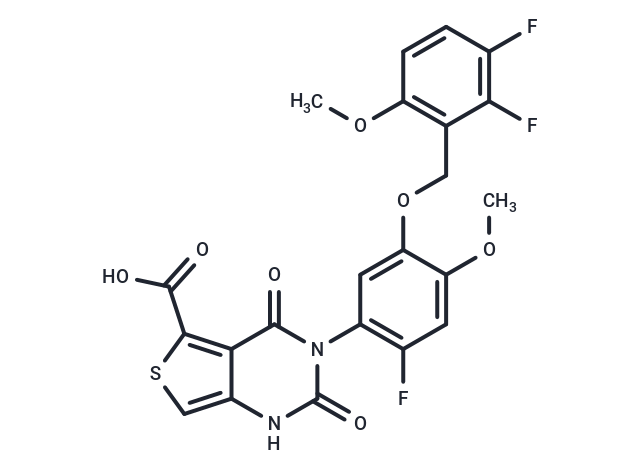
| Smiles | COc1cc(F)c(cc1OCc1c(OC)ccc(F)c1F)-n1c(=O)[nH]c2csc(C(O)=O)c2c1=O |
| Relative Density. | no data available |
| Color | White |
| Appearance | Solid |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 55 mg/mL (108.18 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.