Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Cimlanod is a second-generation Nitroxyl (HNO) donor for heart failure with positive lusitropic and inotropic as well as vasodilatory effects. Cimlanod delivers HNO via pH-dependent chemical breakdown when exposed to the neutral pH environment of the bloodstream.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 25 mg | Inquiry | 6-8 weeks | 6-8 weeks | |
| 50 mg | Inquiry | 6-8 weeks | 6-8 weeks | |
| 100 mg | Inquiry | 6-8 weeks | 6-8 weeks |
| Description | Cimlanod is a second-generation Nitroxyl (HNO) donor for heart failure with positive lusitropic and inotropic as well as vasodilatory effects. Cimlanod delivers HNO via pH-dependent chemical breakdown when exposed to the neutral pH environment of the bloodstream. |
| Synonyms | CXL-1427, BMS-986231 |
| Molecular Weight | 177.18 |
| Formula | C5H7NO4S |
| Cas No. | 1620330-72-4 |
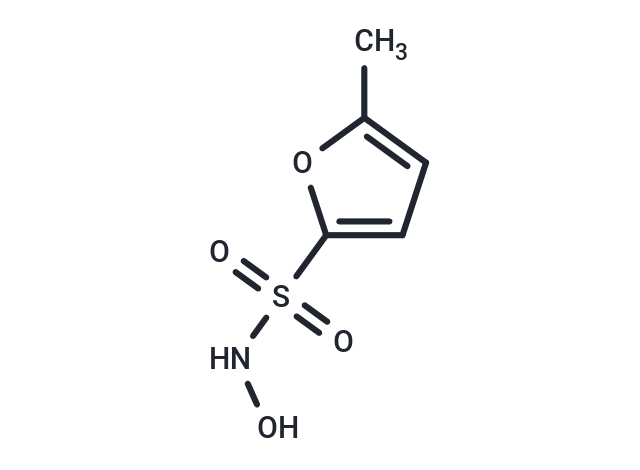
| Smiles | Cc1ccc(o1)S(=O)(=O)NO |
| Relative Density. | 1.475 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 125 mg/mL (705.5 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 3.3 mg/mL (18.63 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.