Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
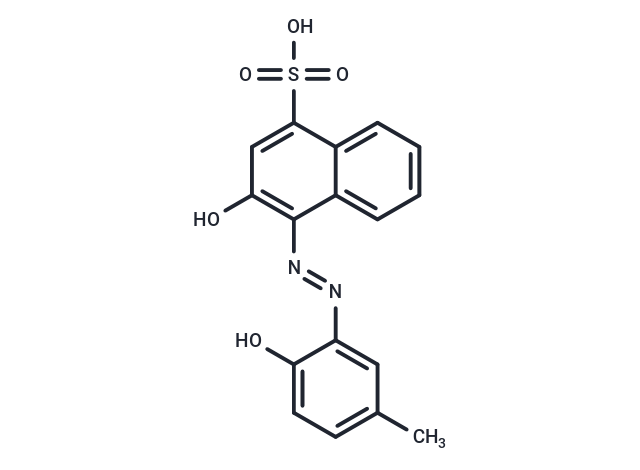
Calmagite is an indicator that can be used to detect magnesium and calcium in various samples.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 500 mg | $29 | Inquiry | Inquiry | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock | In Stock |
| Description | Calmagite is an indicator that can be used to detect magnesium and calcium in various samples. |
| Cell Research | Instructions 1. Dissolution and preparation: Calmagite is usually provided in solid form. When preparing the solution, water or an alcohol-water mixed solvent is usually used for dissolution. The concentration of the solution can be adjusted according to the requirements of the experiment, and the common working concentration range is 0.05% to 1% (w/v). 2. Calcium and magnesium detection (titration): 1. Sample preparation: In water hardness determination or similar analysis, the sample (such as water or other liquids) usually needs to be titrated. 2. Add Calmagite: Add a few drops of Calmagite solution to the sample. Calmagite forms a red complex with calcium ions and a blue complex with magnesium ions, and the specific color depends on the pH value of the solution. 3. Titration with EDTA: In most applications, the sample is titrated with EDTA (ethylenediaminetetraacetic acid) solution, which complexes with calcium and magnesium ions. During the titration process, the color of the solution will change from red (complexed with calcium ions) to blue (complexed with magnesium ions), indicating the presence of these metal ions. 4. Endpoint determination: The endpoint of the titration is usually determined by a color change, indicating that all calcium and magnesium ions have reacted with EDTA. 5. Colorimetric detection of calcium and magnesium: Calmagite can also be used for colorimetric detection. When samples containing calcium and magnesium ions are mixed with Calmagite, the intensity of the color produced can be measured by spectrophotometry to quantify the concentration of calcium and magnesium ions in the sample. Based on the color change, the transition from red to blue can provide a simple visual or instrumental detection method. 6. Effect of pH: When using Calmagite, the pH of the solution is very important. The optimal pH range is usually 9.5 to 10.5. It may be necessary to adjust the pH of the sample using a buffer to ensure proper color formation and accurate results. 7. Cleaning and post-analysis processing: After the experiment is completed, ensure that all glassware and instruments are properly cleaned to avoid cross contamination. Residual dyes on the equipment can be removed using appropriate solvents, such as dilute acids or alcohols. Notes: 1. Calmagite solutions should be stored in a cool and dry place away from direct sunlight. 2. Unused dye solution should avoid repeated freezing and thawing to maintain its stability. The above information is based on published literature. Experimental procedures should be appropriately modified to meet specific research demands. |
| Molecular Weight | 358.37 |
| Formula | C17H14N2O5S |
| Cas No. | 3147-14-6 |
| Smiles | Cc1ccc(O)c(c1)\N=N\c1c(O)cc(c2ccccc12)S(O)(=O)=O |
| Relative Density. | 1.48 g/cm3 |
| Color | Black |
| Appearance | Solid |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 30 mg/mL (83.71 mM), Sonication is recommended. H2O: 7.14 mg/mL (19.92 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 2 mg/mL (5.58 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
H2O/DMSO
DMSO
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.