 Your shopping cart is currently empty
Your shopping cart is currently empty

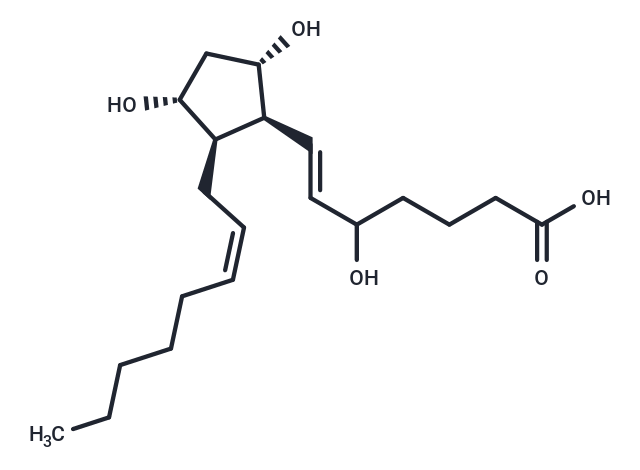
Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation. Although the isoprostanes derived from arachidonic acid are the best characterized, many other polyunsaturated fatty acids can form isoprostanes. (±)5-iPF2α-VI is one of dozens of possible stereo- and regioisomeric isoprostanes which can be formed from arachidonic acid. To date, the most extensively studied of these is 8-isoprostane (8-epi-PGF2α, iPF2α-III). However, 8-isoprostane is a minor isoprostane constituent when compared to some of the other isomers which form in natural conditions of oxidative stress. (±)5-iPF2α-VI is an isoprostane from the unique Type VI class of isoprostanes. This class has been shown to be one of the major isoprostane products, in contrast to 8-isoprostane. In addition to being produced in greater abundance than 8-isoprostane, Type VI isoprostanes form internal lactones, which facilitates their extraction and purification from biological samples.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 10 μg | $213 | 35 days | 35 days | |
| 25 μg | $485 | 35 days | 35 days | |
| 50 μg | $917 | 35 days | 35 days | |
| 100 μg | $1,620 | 35 days | 35 days |
| Description | Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation. Although the isoprostanes derived from arachidonic acid are the best characterized, many other polyunsaturated fatty acids can form isoprostanes. (±)5-iPF2α-VI is one of dozens of possible stereo- and regioisomeric isoprostanes which can be formed from arachidonic acid. To date, the most extensively studied of these is 8-isoprostane (8-epi-PGF2α, iPF2α-III). However, 8-isoprostane is a minor isoprostane constituent when compared to some of the other isomers which form in natural conditions of oxidative stress. (±)5-iPF2α-VI is an isoprostane from the unique Type VI class of isoprostanes. This class has been shown to be one of the major isoprostane products, in contrast to 8-isoprostane. In addition to being produced in greater abundance than 8-isoprostane, Type VI isoprostanes form internal lactones, which facilitates their extraction and purification from biological samples. |
| Synonyms | (±)5-iPF2α-VI |
| Molecular Weight | 354.487 |
| Formula | C20H34O5 |
| Cas No. | 179094-11-2 |
| Smiles | CCCCC\C=C/C[C@H]1[C@H](O)C[C@H](O)[C@H]1\C=C\C(O)CCCC(O)=O |
| Relative Density. | 1.153 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMF: 50 mg/mL (141.05 mM), Sonication is recommended. DMSO: 50 mg/mL (141.05 mM), Sonication is recommended. Ethanol: 50 mg/mL (141.05 mM), Sonication is recommended. PBS (pH 7.2): 0.5 mg/mL (1.41 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
PBS (pH 7.2)/DMF/DMSO/Ethanol
DMF/DMSO/Ethanol
| |||||||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.