 Your shopping cart is currently empty
Your shopping cart is currently empty

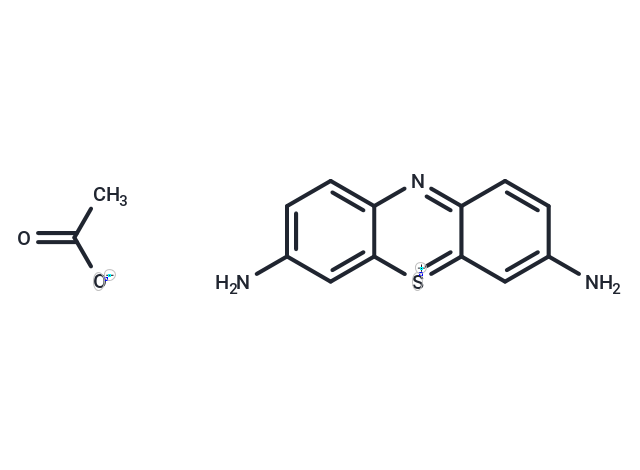
Thionin acetate (Thionine acetate) is a widely used metachromatic cationic dye in histological staining.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 500 mg | $42 | Inquiry | Inquiry | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock | In Stock |
| Description | Thionin acetate (Thionine acetate) is a widely used metachromatic cationic dye in histological staining. |
| Cell Research | 1. Cell staining Experimental steps: 1. Prepare Thionin acetate solution: Dissolve Thionin acetate in an appropriate solvent (usually distilled water or PBS), at a concentration generally between 0.1% and 0.5%. 2. Fix the cells: Apply the cells to be stained onto the slide and fix the cells with an appropriate fixative (such as methanol or formaldehyde) after air-drying. 3. Staining: Add Thionin acetate solution dropwise to the sample. The staining time is usually 5-10 minutes and can be extended as needed. 4. Cleaning: Gently clean the sample with distilled water to remove excess dye. 5. Microscope observation: Use a microscope to observe the cell staining effect. Thionin acetate will produce a purple-blue staining effect to highlight the cell nucleus. 2. Staining tissue sections Experimental steps: 1. Prepare Thionin acetate solution: Configure the solution as needed, the concentration is generally 0.1% to 0.5%. 2. Preparation of tissue sections: Fix the tissue sections on a slide, fix them with fixative (such as 10% formalin solution), and clear them after conventional dehydration. 3. Staining: Add Thionin acetate solution dropwise to the sections. The staining time is usually 5-15 minutes. The time can be adjusted appropriately depending on the tissue type. 4. Clean and dehydrate: Clean the sections with distilled water and then dehydrate. 5. Seal and observation: Seal the tablet with an appropriate sealant, and then observe the staining effect under a microscope. 3. Neural tissue staining: Experimental steps: 1. Prepare Thionin acetate solution: The solution concentration is usually 0.1% to 0.5%. 2. Brain tissue section processing: Perform routine fixation, sectioning and dehydration of brain tissue. 3. Staining: Add Thionin acetate solution dropwise to the sections. The staining time is usually 10-20 minutes, which is suitable for observing the details of the neural tissue. 4. Clean and seal the sections: Wash the sections with water or PBS, then seal the sections and observe the brain tissue structure using a microscope. Notes: 1. Concentration control: Too high concentration of Thionin acetate may lead to excessive staining, so the staining concentration needs to be adjusted according to experimental needs. 2. Staining time: The staining time should be optimized according to the type of tissue and experimental needs to avoid excessive staining or excessive background due to excessive staining time. 3. Tissue pretreatment: Before performing Thionin acetate staining, it is necessary to ensure that the tissue sections are properly fixed and dehydrated to ensure the staining effect. 4. Microscopic observation: Use an optical microscope to observe the sample. The nucleus stained by Thionin acetate is purple-blue, which is suitable for observing cell and tissue structure. The above information is based on published literature. Experimental procedures should be appropriately modified to meet specific research demands. |
| Synonyms | Thionine acetate |
| Molecular Weight | 287.34 |
| Formula | C14H13N3O2S |
| Cas No. | 78338-22-4 |
| Smiles | CC([O-])=O.Nc1ccc2nc3ccc(N)cc3[s+]c2c1 |
| Relative Density. | 0.82?g/mL?at 25?°C(lit.) |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 41.67 mg/mL (145.02 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| In Vivo Formulation | 10% DMSO+40% PEG300+5% Tween-80+45% Saline: 2 mg/mL (6.96 mM), Sonication is recommended. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.