Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
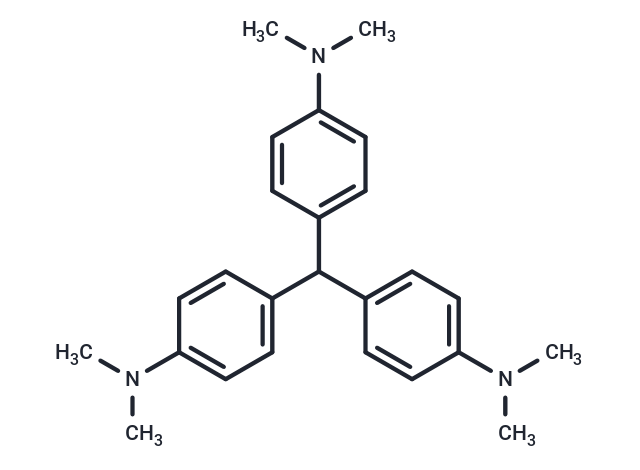
Leucocrystal Violet is a triphenylmethane dye.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 1 g | $29 | - | In Stock | |
| 1 mL x 10 mM (in DMSO) | $30 | In Stock | In Stock |
| Description | Leucocrystal Violet is a triphenylmethane dye. |
| Cell Research | Instructions I. Reagent preparation: Dissolve it in an appropriate solvent (such as water or buffer solution). Depending on the experimental requirements, the common concentration range is 0.1-1 mM. II. Operation steps 1. Sample preparation: The sample can be water, soil, air or biological fluids. If the sample contains impurities or other metal ions, it may need to be pretreated or diluted to reduce interference. 2. Reaction steps: 1) Take a certain volume of sample solution (such as environmental water samples, biological fluids, etc.) and add Leucocrystal Violet solution. 2) Usually, the reaction is carried out under acidic conditions, and the pH value of the solution is adjusted to the optimal range of the reaction using an acid of appropriate concentration (such as hydrochloric acid, acetic acid, etc.). 3) The sample reacts with Leucocrystal Violet at room temperature for a certain time (usually 10-30 minutes) to form a purple complex. 3. Measurement: 1) Use a spectrophotometer to measure the absorbance (OD value) at an appropriate wavelength (usually 590 nm). The concentration of the purple complex generated after the reaction of antimony and LCV is proportional to the absorbance. 2) According to the standard curve, calculate the concentration of antimony in the sample. 4. Data analysis: 1) According to the absorbance value of the sample, the concentration of antimony is estimated through the standard curve. 2) Blank control experiments and comparisons between samples may be required to ensure the accuracy of the results. Notes: 1. Interfering substances: Other metal ions (such as lead, chromium, etc.) in the sample may react with Leucocrystal Violet and affect the results. Therefore, appropriate separation or suppression of interference from other metals may be required before the experiment. 2. pH control: The pH value of the reaction has an important influence on sensitivity and stability. The pH value of the reaction system should be ensured to be within the optimal range. 3. Stability: The purple complex after the reaction should be measured as soon as possible, because the stability of the complex is poor under light or high temperature and may degrade. The above information is based on published literature. Experimental procedures should be appropriately modified to meet specific research demands. |
| Synonyms | Leucogentian violet |
| Molecular Weight | 373.53 |
| Formula | C25H31N3 |
| Cas No. | 603-48-5 |
| Smiles | CN(C)c1ccc(cc1)C(c1ccc(cc1)N(C)C)c1ccc(cc1)N(C)C |
| Relative Density. | 1.077g/cm3 |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||
| Solubility Information | DMSO: 3.74 mg/mL (10.01 mM), Sonication is recommended. H2O: < 0.1 mg/mL (insoluble) | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.