 Your shopping cart is currently empty
Your shopping cart is currently empty

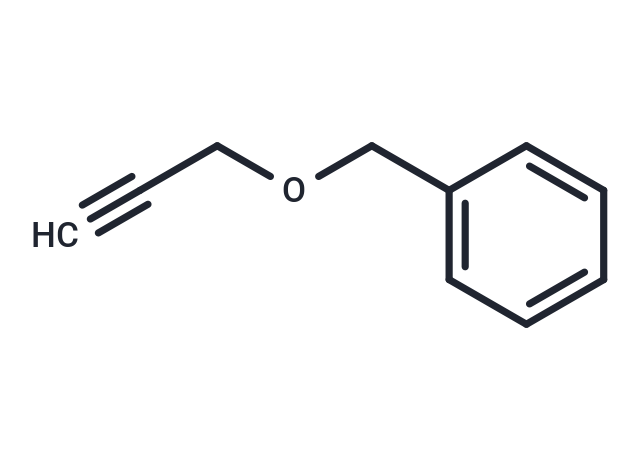
Benzyl propargyl ether is a versatile synthetic building block characterized by the linear formula C₆H₅CH₂OCH₂C≡CH and defined by its terminal alkyne functionality, which enables participation in diverse carbon–carbon and carbon–heteroatom bond-forming reactions. Benzyl propargyl ether is widely used in the synthesis of substituted carbocyclic aromatic frameworks, pharmaceutical intermediates, and advanced polymeric materials, Benzyl propargyl ether also serves as a valuable precursor in copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) click chemistry for rapid assembly of functional dendrimers and macromolecules. in carbohydrate chemistry,Benzyl propargyl ether functions as a sterically minimal O-protecting group, and its use at the O-2 position of 4,6-O-benzylidene-protected mannosyl donors enables exceptionally high β-selectivity during challenging glycosylation reactions, supporting efficient synthesis of complex oligosaccharides and glycans.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 mg | $50 | Inquiry | Inquiry | |
| 10 mg | $70 | Inquiry | Inquiry | |
| 25 mg | $113 | Inquiry | Inquiry | |
| 50 mg | $163 | Inquiry | Inquiry | |
| 100 mg | $239 | Inquiry | Inquiry |
| Description | Benzyl propargyl ether is a versatile synthetic building block characterized by the linear formula C₆H₅CH₂OCH₂C≡CH and defined by its terminal alkyne functionality, which enables participation in diverse carbon–carbon and carbon–heteroatom bond-forming reactions. Benzyl propargyl ether is widely used in the synthesis of substituted carbocyclic aromatic frameworks, pharmaceutical intermediates, and advanced polymeric materials, Benzyl propargyl ether also serves as a valuable precursor in copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) click chemistry for rapid assembly of functional dendrimers and macromolecules. in carbohydrate chemistry,Benzyl propargyl ether functions as a sterically minimal O-protecting group, and its use at the O-2 position of 4,6-O-benzylidene-protected mannosyl donors enables exceptionally high β-selectivity during challenging glycosylation reactions, supporting efficient synthesis of complex oligosaccharides and glycans. |
| Synonyms | ((Prop-2-yn-1-yloxy)methyl)benzene |
| Molecular Weight | 146.19 |
| Formula | C10H10O |
| Cas No. | 4039-82-1 |
| Smiles | C#CCOCC=1C=CC=CC1 |
| Color | Transparent |
| Appearance | Liquid |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year |
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.