Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
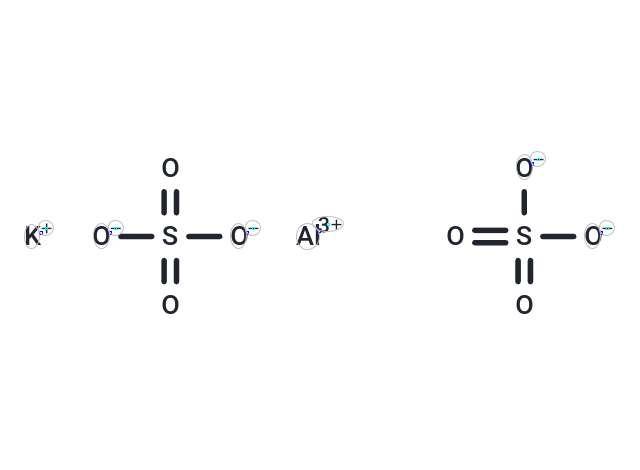
Aluminum potassium sulfate (Potassium Aluminium Sulfate) is used in the study of severe acute respiratory syndrome and may be used in the prevention and treatment of cancer.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 10 g | $40 | - | In Stock |
| Description | Aluminum potassium sulfate (Potassium Aluminium Sulfate) is used in the study of severe acute respiratory syndrome and may be used in the prevention and treatment of cancer. |
| In vivo | Aluminum potassium sulfate (APS) 1 g.kg-1.d-1 for 60 d induced learning and memory deficits in mice. APS 1 g.kg-1 increased blood-Al only after 30 d. After 60 d, STL, ACh content and ChAT activity decreased by 46.4%, 8.5%, and 22.6%, respectively. These parameters decreased by 50%, 11.1%, and 27.8%, respectively, with increased Al in blood and brain, after 90 d.[2] In ethylcholine mustard aziridium chloride (AF64A) treated mice, APS 1 g.kg-1 only increased blood and brain-Al.[2] |
| Synonyms | Potassium Aluminium Sulfate, APS |
| Molecular Weight | 258.19 |
| Formula | AlKO8S2 |
| Cas No. | 10043-67-1 |
| Smiles | O=S([O-])([O-])=O.O=S([O-])([O-])=O.[Al+3].[K+] |
| Color | White |
| Appearance | Solid |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 27.5 mg/mL (106.51 mM), Sonication is recommended. DMSO: < 1 mg/mL (insoluble or slightly soluble) | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
H2O
| ||||||||||||||||||||||||||||||||||||
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.