 Your shopping cart is currently empty
Your shopping cart is currently empty

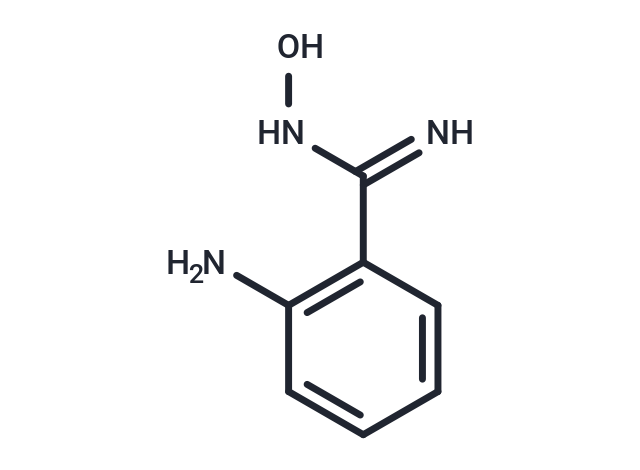
2-Amino benzamidoxime (ABAO compound 6) swiftly reacts with aldehydes in aqueous solution to form a stable 1,2-dihydroquinazoline-3-oxide (dihydroquinazoline derivative). The reaction involves the formation of a Schiff base as the rate-determining step, followed by rapid intramolecular cyclization. The reaction rate is pH-dependent, indicating that protonated benzamidoxime acts as an internal general acid in Schiff base formation. Substituents on the aromatic ring can increase the basicity of the aromatic amine, accelerating the reaction. The reactive properties of 2-Amino benzamidoxime suggest its potential as a platform for developing new bioconjugation strategies, fluorescent probes, and post-translational diversification of genetically encoded libraries.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 10 mg | Inquiry | 10-14 weeks | 10-14 weeks | |
| 50 mg | Inquiry | 10-14 weeks | 10-14 weeks |
| Description | 2-Amino benzamidoxime (ABAO compound 6) swiftly reacts with aldehydes in aqueous solution to form a stable 1,2-dihydroquinazoline-3-oxide (dihydroquinazoline derivative). The reaction involves the formation of a Schiff base as the rate-determining step, followed by rapid intramolecular cyclization. The reaction rate is pH-dependent, indicating that protonated benzamidoxime acts as an internal general acid in Schiff base formation. Substituents on the aromatic ring can increase the basicity of the aromatic amine, accelerating the reaction. The reactive properties of 2-Amino benzamidoxime suggest its potential as a platform for developing new bioconjugation strategies, fluorescent probes, and post-translational diversification of genetically encoded libraries. |
| Synonyms | ABAO |
| Molecular Weight | 151.17 |
| Formula | C7H9N3O |
| Cas No. | 16348-49-5 |
| Smiles | N=C(NO)C=1C=CC=CC1N |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year |
Dissolve 2 mg of the compound in 100 μL DMSO![]() to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
to obtain a stock solution at a concentration of 20 mg/mL . If the required concentration exceeds the compound's known solubility, please contact us for technical support before proceeding.
1) Add 100 μL of the DMSO![]() stock solution to 400 μL PEG300
stock solution to 400 μL PEG300![]() and mix thoroughly until the solution becomes clear.
and mix thoroughly until the solution becomes clear.
2) Add 50 μL Tween 80 and mix well until fully clarified.
3) Add 450 μL Saline,PBS or ddH2O![]() and mix thoroughly until a homogeneous solution is obtained.
and mix thoroughly until a homogeneous solution is obtained.
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.