 Your shopping cart is currently empty
Your shopping cart is currently empty


Oil Red O Staining Solution
Copy Product Info| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 30 mL | $63 | In Stock | In Stock |
 Product Information
Product Information
| Oil Red O Staining Solution | Specifications |
|---|---|
| Ingredient | Oil Red O |
| CAS | 1320-06-5 |
| Conc. | 0.5% |
| Solvent | Isopropanol |
 Features
Features
1.Strong specificity for lipid droplet labeling.
2.Easy to use.
3.Good staining performance.
4.High cost-effectiveness.
 Application
Application
-
Adipocyte differentiation assay
-
Hepatocyte lipid metabolism model
-
Immune cell lipid phagocytosis assay
-
Atherosclerotic plaque detection
 Preparation of Working Solution
Preparation of Working Solution
Dilute the Oil Red O stock solution with ddH₂O at a volume ratio of 3:2, then filter through a 0.45 µm membrane to prepare the staining working solution. The freshly prepared working solution should appear burgundy in color without any precipitate. It is recommended to prepare the working solution freshly before use to avoid precipitation caused by prolonged storage.
 Instructions
Instructions
1. Staining of Adherent Cells
(1) Remove the cell culture medium, wash once with PBS, and add an appropriate amount of 4% paraformaldehyde solution or 10% formaldehyde solution to fix for 10 min.
(2) Discard the fixative, wash twice with PBS, and incubate with 60% isopropanol for 20-30 s.
(3) Remove the 60% isopropanol, add freshly prepared Oil Red O working solution, and stain for 10-20 min.
(4) Discard the staining solution, rinse with 60% isopropanol for 10–20 s until the background is clear. Wash with ddH2O 2–5 times until no excess stain leaches out.
(5) Add hematoxylin solution to counterstain the nuclei for 1-3 min. Remove the stain and rinse gently with running water (avoid direct water flow onto the cells during operation).
(6) Cover the cells with ddH2O, transfer them to a microscope, and take photographs.
2. Staining of Cell Smears
(1)Prepare fresh bone marrow or blood smears and allow them to air dry naturally at room temperature. Fix with 4% paraformaldehyde for 10-15 minutes, then rinse twice with ddH2O.
(2)Add freshly prepared Oil Red O working solution and stain for 10-20 minutes.
(3)Remove the staining solution, rinse with 60% isopropanol for 10-20 seconds, and then wash with ddH2O 2-5 times.
(4)Add hematoxylin staining solution to counterstain the nuclei for 1-3 minutes. After discarding the staining solution, gently rinse with running water (avoid direct flushing of the cells).
(5)(Optional) Mount the smear with an aqueous mounting medium, then observe and photograph under a microscope.
 Storage
Storage
Store at room temperature, protected from light. Valid for one year.
 Precautions
Precautions
1.The staining time for Oil Red O is flexible.
2.Oil Red O solution is a saturated solution. When stored at room temperature or 4ºC, a small amount of precipitation or insoluble material adhering to the bottle wall may occur, which does not affect its use.
3.During Oil Red O staining, evaporation of the staining solution should be avoided, as it may cause precipitation and lead to background staining.
4.Frozen sections for Oil Red O staining sould be relatively thick, generally 10–15 µm.
5.Oil Red O staining results cannot be preserved for a long time; samples should be observed promptly.
6.The product is for R&D use only, not for diagnostic procedures, food, drug, household or other uses.
7.Please wear a lab coat and disposable gloves.
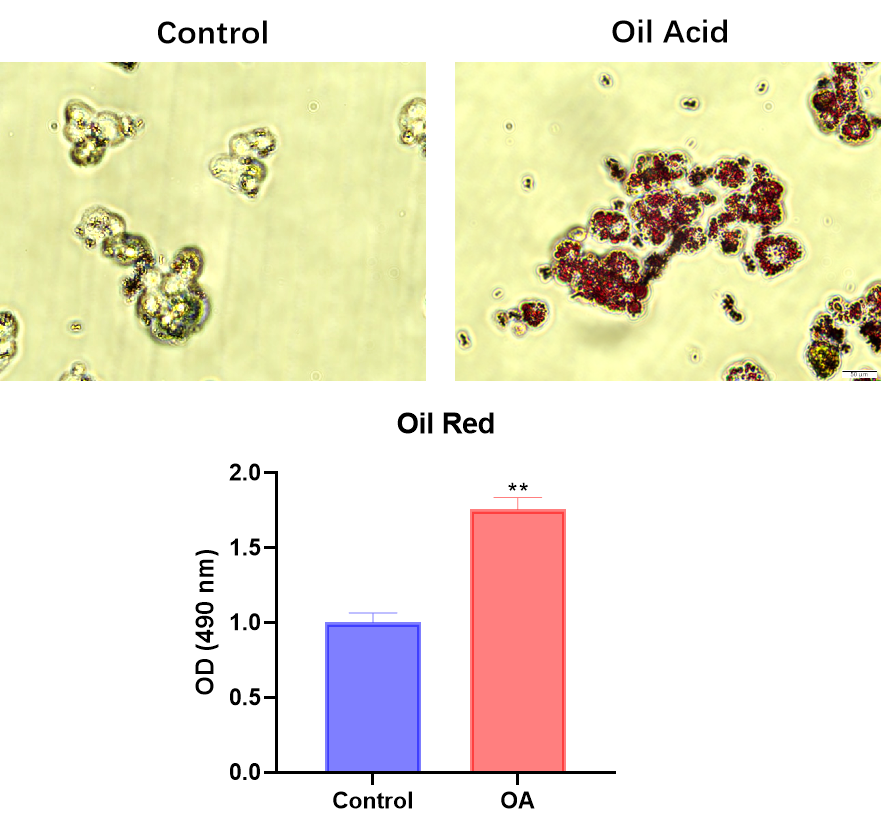
 Staining Performance
Staining Performance
- Oil acid induces the differentiation of HepG2 cells into adipocytes
 Instruction Manual
Instruction Manual
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.



