Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
Anti-Phospho-NFkB p100 (Ser870) Polyclonal Antibody is a Rabbit antibody targeting Phospho-NFkB p100 (Ser870). Anti-Phospho-NFkB p100 (Ser870) Polyclonal Antibody can be used in IF,IHC,WB.
| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 50 μL | $216 | 7-10 days | 7-10 days | |
| 100 μL | $316 | 7-10 days | 7-10 days |
| Description | Anti-Phospho-NFkB p100 (Ser870) Polyclonal Antibody is a Rabbit antibody targeting Phospho-NFkB p100 (Ser870). Anti-Phospho-NFkB p100 (Ser870) Polyclonal Antibody can be used in IF,IHC,WB. |
| Synonyms | p-NFkB p100 (Ser870), p-NFkB p100 (S870), NFkB p100 (p-Ser870), NFkB p100 (p-S870) |
| Ig Type | IgG |
| Reactivity | Human,Mouse,Rat |
| Verified Activity | 1. Immunofluorescence staining of methanol-fixed HeLa cells using NF-κB p100(phospho-Ser870) antibody (TMAC-02826, Red). 2. Immunohistochemical analysis of paraffin-embedded human breast carcinoma tissue using NF-κB p100(phospho- Ser870) antibody (TMAC-02826). 3. Western blot analysis of extract from MDA-MB-435 cells untreated or treated with TNF-α (20ng/ml, 5min) using NF-κB p100(phospho-Ser870) antibody (TMAC-02826). 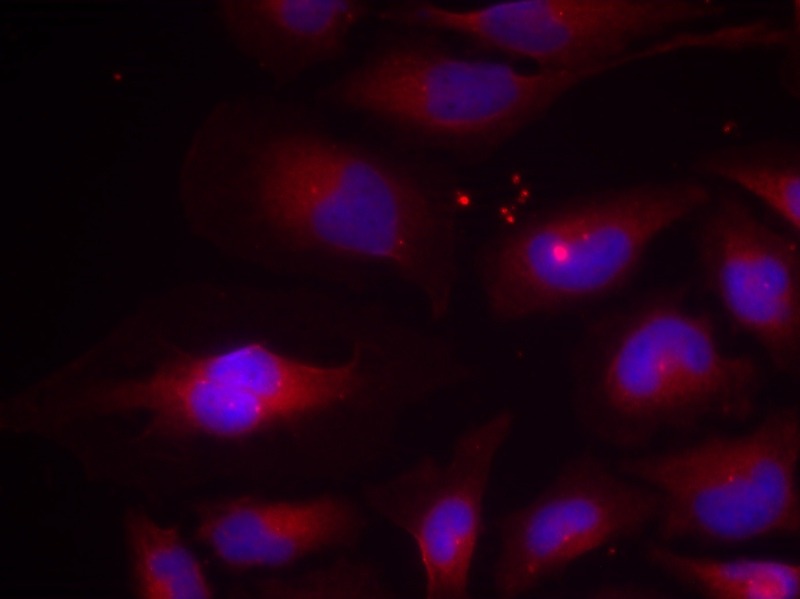 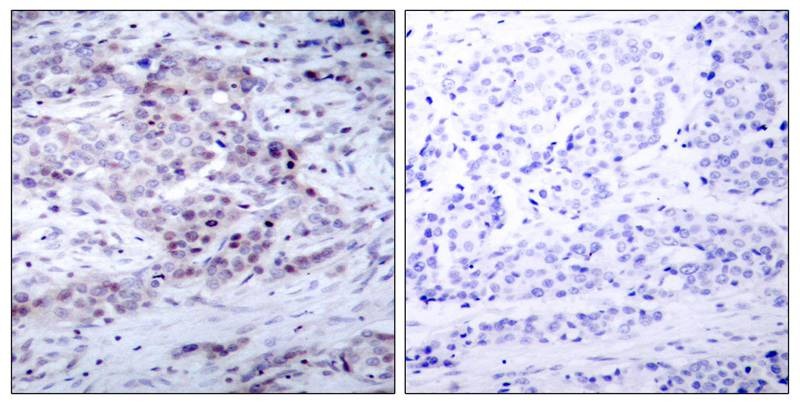 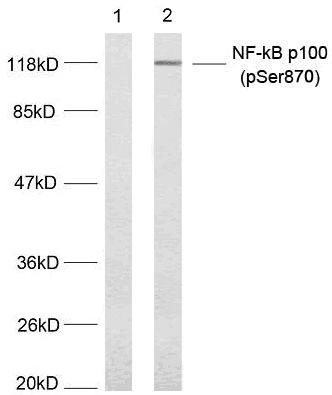 |
| Application | |
| Antibody Type | Polyclonal |
| Host Species | Rabbit |
| Construction | Polyclonal Antibody |
| Purification | Antibodies were produced by immunizing rabbits with synthetic phosphopeptide and KLH conjugates. Antibodies were purified by affinity-chromatography using epitope-specific phosphopeptide. Non-phospho specific antibodies were removed by chromatogramphy using non-phosphopeptide. |
| Appearance | Liquid |
| Formulation | Supplied at 1.0mg/mL in phosphate buffered saline (without Mg2+ and Ca2+), pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol. |
| Research Background | NF-kappa-B is a pleiotropic transcription factor present in almost all cell types and is the endpoint of a series of signal transduction events that are initiated by a vast array of stimuli related to many biological processes such as inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis. NF-kappa-B is a homo- or heterodimeric complex formed by the Rel-like domain-containing proteins RELA/p65, RELB, NFKB1/p105, NFKB1/p50, REL and NFKB2/p52. The dimers bind at kappa-B sites in the DNA of their target genes and the individual dimers have distinct preferences for different kappa-B sites that they can bind with distinguishable affinity and specificity. Different dimer combinations act as transcriptional activators or repressors, respectively. NF-kappa-B is controlled by various mechanisms of post-translational modification and subcellular compartmentalization as well as by interactions with other cofactors or corepressors. NF-kappa-B complexes are held in the cytoplasm in an inactive state complexed with members of the NF-kappa-B inhibitor (I-kappa-B) family. In a conventional activation pathway, I-kappa-B is phosphorylated by I-kappa-B kinases (IKKs) in response to different activators, subsequently degraded thus liberating the active NF-kappa-B complex which translocates to the nucleus. In a non-canonical activation pathway, the MAP3K14-activated CHUK/IKKA homodimer phosphorylates NFKB2/p100 associated with RelB, inducing its proteolytic processing to NFKB2/p52 and the formation of NF-kappa-B RelB-p52 complexes. The NF-kappa-B heterodimeric RelB-p52 complex is a transcriptional activator. The NF-kappa-B p52-p52 homodimer is a transcriptional repressor. NFKB2 appears to have dual functions such as cytoplasmic retention of attached NF-kappa-B proteins by p100 and generation of p52 by a cotranslational processing. The proteasome-mediated process ensures the production of both p52 and p100 and preserves their independent function. p52 binds to the kappa-B consensus sequence 5'-GGRNNYYCC-3', located in the enhancer region of genes involved in immune response and acute phase reactions. p52 and p100 are respectively the minor and major form; the processing of p100 being relatively poor. Isoform p49 is a subunit of the NF-kappa-B protein complex, which stimulates the HIV enhancer in synergy with p65. Dobrzanski P., Ryseck R.P., Bravo R.EMBO J. 13:4608-4616(1994) Betts J.C., Nabel G.J.Mol. Cell. Biol. 16:6363-6371(1996) |
| Conjucates | Unconjugated |
| Others Formats | Phospho |
| Immunogen | Peptide sequence around phosphorylation site of serine 870(Y-G-S(p)-Q-S) derived from Human NFκB-p100 |
| Antigen Species | human |
| Uniprot ID |
| Stability & Storage | Store at -20°C or -80°C for 12 months. Avoid repeated freeze-thaw cycles. |
| Transport | Shipping with blue ice. |
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.